In Australia, there are two health care systems:
A clearly defined public hospital system manages the health care needs of the entire population. Access is not dependent on socioeconomic status. This system deals with the majority of patients requiring emergency care and provides education for medical and nursing staff.
A private hospital system manages patients with private health insurance. This system is predominantly led by medical specialists, with the recent introduction of junior medical staff onto the wards. The proportion of the Australian population with private medical insurance has increased from 33.6% to 43.0% over the past 10 years.1
There have been few meaningful comparisons of outcomes for patients managed in these two systems. The aim of our study was to determine whether treatment in private versus public hospitals was an independent predictor of improved survival outcomes in patients with colorectal cancer. Risk was stratified according to the covariates known to influence survival in patients with colorectal cancer.2
Tumour stage was classified according to the current American Joint Committee on Cancer guidelines.3 The histopathological report for each patient was reviewed and the information recorded. Anatomical site of the tumour was ascertained from the pathology reports and crosschecked with information from admission and procedure records. Rectal carcinomas were defined as originating within 12 cm of the anal verge. If cancer location could not be clearly defined, then it was coded as colonic.
The 2001 Australian Bureau of Statistics report on Socio-Economic Indexes for Areas was used to gain information on indexes of community disadvantage.4 For each patient, we determined the values for the index of relative socioeconomic advantage/disadvantage, the index of economic resources, and the index of education and occupation. A frequency distribution was then performed, and the patients were divided into quintiles. These quintiles were used as an ordinal scale of social disadvantage.
The Cox proportional hazards model was used to define the relationship between a number of covariates and overall and cancer-specific survival. The covariates included: age, sex, site of cancer (colon versus rectum), cancer stage (ie, I, II, III, and IV),3 use of chemotherapy, use of radiotherapy, public hospital versus private hospital, the index of relative socioeconomic advantage/disadvantage. All the variables were categorical apart from age (years), which was analysed as a continuous variable. All variables were entered into the analysis using the forced-entry method. Significance was defined as the probability of a type 1 error of less than 5%. The results are presented as relative risk (RR) and 95% confidence intervals. The likelihood statistic was used as an overall test of the Cox regression model.
Between 1993 and 2003, 5809 patients with colorectal cancer were managed in Western Australia. Of these, 1523 patients (26%) were treated in private hospitals. Demographic data for the two groups are given in Box 1. Patients treated in private hospitals were younger, and had a lower 30-day postoperative mortality rate. In addition, they had a higher index of relative socioeconomic advantage/disadvantage, a higher index of economic resources, and a higher index of education and occupation.
Public hospital patients were more likely to have rectal cancers and Stage IV disease (Box 2). A higher proportion of private hospital patients had unknown staging (7% v 0.3%, Box 2). Most of these patients were diagnosed from a biopsy and did not undergo surgery in either hospital system. They most likely represent patients with advanced disease. Patients in private hospitals were significantly more likely to receive chemotherapy, especially if they had Stage II or III colon cancers. When considering only patients under 75 years of age, there was still a significantly higher use of chemotherapy in private hospitals. There was no difference between public and private hospital patients in the use of radiotherapy in those with rectal cancer.
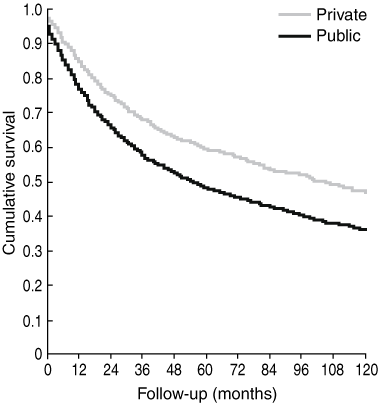
Patients treated in private hospitals had significantly better 5-year overall survival and cancer-specific survival rates when compared with patients treated in public hospitals (Box 3 and Box 4). This survival advantage was seen in all stages of disease except for the cancer-specific survival in patients with Stage I disease (Box 3).
A Cox proportional hazards regression analysis was then performed to evaluate the influence of a number of covariates on overall and cancer-specific survival rates (Box 5). Independent predictors of improved overall survival included: treatment in a private hospital, diagnosis at a younger age, female sex, and cancer stage. Independent predictors of cancer-specific survival included: treatment in a private hospital, diagnosis at a younger age, and cancer stage.
Clearly, not all of the potential factors that impact on survival could be adjusted for. This analysis was unable to clearly determine prognostic factors such as mode of presentation (ie, emergency v elective treatment), general health of the patient (eg, smoking status and American Society of Anesthesiologists [ASA] score — a subjective categorisation of patients into five subgroups by preoperative physical fitness), and quality of surgery.2
Why were there improved survival outcomes for patients treated in a private hospital? This finding may reflect improved standards of surgery; in general, surgeons performing high volumes of procedures have improved patient survival outcomes.5 There was also increased access to chemotherapy in private hospitals. This was especially noted in patients with Stage II and III disease and appeared to be independent of patient age. There is now acceptance of the use of chemotherapy as standard treatment for Stage III disease,2 yet in public hospitals only 45% of patients with Stage III disease who were less than 75 years of age received chemotherapy. This compares with 67% of similar patients in private hospitals. This low rate of compliance with accepted clinical standards is a point of significant concern.
There have been previous attempts at defining the relationship between socioeconomic status, private health insurance, and outcomes for cancer. Within Western Australia, a population-based study by Hall et al6 of patients with colorectal cancer diagnosed between 1982 and 2001 (n = 14 587) attempted to address these issues. This study found that socioeconomic and location status, as well as private health insurance, did not influence overall survival. In Hall et al’s study, patients’ data were accessed directly from the Western Australian Linked Database, and no attempt was made to individually validate the pathology records and check on cancer staging. Patients with carcinoid tumours, adenosquamous and squamous carcinomas of the colon and rectum were included. The study also covered a time period when there was little use of adjuvant therapy. In contrast, our study included only patients with adenocarcinoma of the colon and rectum who had undergone a procedure or a biopsy; selection was from the pathology services; the results were all checked; and staging information was gained. This information was then crosschecked with the Cancer Registry and the Western Australian Linked Database. All diagnoses and treatments were validated.
The influence of socioeconomic status on cancer survival has been studied in other population centres in Australia and overseas. A regional study of patients with lung cancer in New South Wales (n = 526) found similar patterns of care and survival outcomes despite variation in the socioeconomic indexes.7 A population-based study in Norway on cancer outcomes (n = 98 992) found that survival improved with increasing average education level, and that this appeared to be due to earlier diagnosis.8 In contrast, in our study, indexes of socioeconomic advantage/disadvantage, economic resources, and education and occupation were not significant determinants of survival in patients with colorectal cancer. Moorin and Holman9 also used the Western Australian Linked Database to study the effects of socioeconomic status on accessibility to services for patients who had died of cancer between 1997 and 2000. They concluded that there was inequality between patients who were privately insured compared with those with no insurance who were relying on the public hospital system. Private patients had greater access to hospital resources.9
Within the United Kingdom there have been a number of population- and regional-based studies evaluating the association between social disadvantage and survival outcomes for patients with colorectal cancer. In contrast to Australia, fewer patients in the UK have private health insurance (11% in 2006 v 43% in Australia).10 Several large UK studies have shown that deprivation predicts a worse survival outcome for patients with colorectal cancer.11-14 These findings are usually significant on univariate analysis; however, when other independent predictors of survival outcomes are included (ie, cancer stage, ASA score, urgency of surgery), then deprivation seems less important. In general, deprived patients presented with more advanced disease and were less fit. Not all UK studies have shown an association between disadvantage and poorer outcomes for patients with colorectal cancer. One review of 603 consecutive patients with colorectal cancer within one district found no relationship between patient socioeconomic status and survival outcome.15
1 Demographic data for patients managed in public and private hospitals in Western Australia (1993–2003)
2 Data on colorectal cancer in patients managed in public and private hospitals in Western Australia (1993–2003)
3 The 5-year overall survival and cancer-specific survival rates for patients with various stages of colorectal cancer managed in Western Australia (1993–2003) — results for public versus private hospitals
4 Comparison of overall survival rates of patients treated for colorectal cancer in public versus private hospitals in Western Australia (1993–2003)
- Melinda Morris1
- Barry Iacopetta2
- Cameron Platell3
- School of Surgery and Pathology, University of Western Australia, Perth, WA.
We would like to thank Dr Timothy Threlfall of the Western Australian Cancer Registry for his assistance in providing mortality data for the study.
None identified.
- 1. Private Health Insurance Administration Council. Percentage of Australians covered by private health insurance. http://www.phiac.gov.au /statistics/membershipcoverage/hosquar.htm (accessed Feb 2007).
- 2. Australian Cancer Network Colorectal Cancer Guidelines Revision Committee. Clinical practice guidelines for the prevention, early detection and management of colorectal cancer (CRC). Sydney: The Cancer Council Australia and Australian Cancer Network, 2005. http://www.cancer.org.au/content.cfm?randid=408243 (accessed Feb 2007).
- 3. American Joint Committee on Cancer. AJCC cancer staging manual. 6th ed. New York: Springer, 2002: 113-124.
- 4. Australian Bureau of Statistics. Information paper: census of population and housing — socio-economic indexes for areas, Australia, 2001. Canberra: ABS, 2003. (Catalogue No. 2039.0.)
- 5. Committee on Quality of Health Care in America and the National Cancer Policy Board. Interpreting the volume–outcome relationship in the context of health care quality. Washington: Institute of Medicine, 2000.
- 6. Hall SE, Holman CD, Platell C, et al. Colorectal cancer surgical care and survival: do private health insurance, socioeconomic and locational status make a difference? ANZ J Surg 2005; 75: 929-935.
- 7. Hui AC, Vinod SK, Jalaludin BB, et al. Socio-economic status and patterns of care in lung cancer. Aust N Z J Public Health 2005; 29: 372-377.
- 8. Kravdal O. Does place matter for cancer survival in Norway? A multilevel analysis of the importance of hospital affiliation and municipality socio-economic resources. Health Place 2006; 12: 527-537.
- 9. Moorin RE, Holman CD. The effects of socioeconomic status, accessibility to services and patient type on hospital use in Western Australia: a retrospective cohort study of patients with homogenous health status. BMC Health Serv Res 2006; 6: 74.
- 10. Foubister T, Thomson S, Mossialos E, McGuire A. Private medical insurance in the United Kingdom. World Health Organization, Regional Office for Europe, 2006. http://www.euro.who.int/InformationSources/Publications/Catalogue/20060602_1 (accessed Feb 2007).
- 11. Jeffreys M, Rachet B, McDowell S, et al. Survival from rectal and anal cancers in England and Wales, 1986-2001. Eur J Cancer 2006; 42: 1434-1440.
- 12. Smith JJ, Tilney HS, Heriot AG, et al. Social deprivation and outcomes in colorectal cancer. Br J Surg 2006; 93: 1123-1131.
- 13. Wrigley H, Roderick P, George S, et al. Inequalities in survival from colorectal cancer: a comparison of the impact of deprivation, treatment, and host factors on observed and cause specific survival. J Epidemiol Community Health 2003; 57: 301-309.
- 14. Hole DJ, McArdle CS. Impact of socioeconomic deprivation on outcome after surgery for colorectal cancer. Br J Surg 2002; 89: 586-590.
- 15. Lyratzopoulos G, Sheridan GF, Michie HR, et al. Absence of socioeconomic variation in survival from colorectal cancer in patients receiving surgical treatment in one health district: cohort study. Colorectal Dis 2004; 6: 512-517.





Abstract
Objective: To determine whether treatment in a private versus public hospital was an independent predictor of survival outcomes in patients with colorectal cancer.
Design: Retrospective, population-based study.
Setting: Tertiary care hospitals.
Participants: All patients diagnosed with colorectal cancer in Western Australia between 1993 and 2003.
Interventions: Management in private versus public hospitals.
Main outcome measures: Overall survival and cancer-specific survival rates.
Results: 5809 patients were treated for colorectal cancer. Of these, 1523 (26%) were managed in private hospitals. The 5-year overall survival rates for private and public hospital patients were 59.4% (95% CI, 56.9%–61.9%) and 48.6% (95% CI, 47.0%–50.2%), respectively. Significant independent predictors of overall survival were: treatment in a private hospital (P = 0.0001; relative risk [RR], 0.764; 95% CI, 0.696–0.839); younger age (P = 0.0001; RR, 1.032; 95% CI, 1.029–1.036); male sex (P = 0.001; RR, 1.148; 95% CI, 1.068–1.234); and cancer stage (eg, Stage II: P = 0.0001; RR, 1.508; 95% CI, 1.316–1.729).
Conclusions: Treatment in a private hospital was a significant independent predictor of survival outcomes. Further validation of these results would have a significant bearing on how we approach health care delivery for patients with colorectal cancer.