Clinical record
Examination showed a painless, full-thickness 4 cm × 3 cm ulcer with a granulating base and raised edges on the patient’s forearm (Figure A). There was no regional lymphadenopathy. Tissue impression smears and multiple 3 mm punch biopsies from the ulcer edge confirmed the presence of inflammatory cells and necrotising granulomas in the dermis. Despite prolonged examination of Giemsa-stained sections, no intracellular parasites were detected. An ulcer edge biopsy sent for polymerase chain reaction (PCR) analysis was negative for Leishmania DNA. However, Leishmania promastigotes were identified on Day 7 from culture of an ulcer biopsy on Novy–McNeal–Nicolle medium (Figure B). PCR analysis confirmed the presence of L. braziliensis promastigotes. Specifically, DNA was extracted from a tissue biopsy and from cultured promastigotes using a DNA Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. A PCR restriction fragment length polymorphism (RFLP) analysis targeting both the end of the ribosomal small subunit and the whole internal transcribed spacer 1 region was performed, as described elsewhere.1,2 Both techniques gave banding patterns consistent with L. braziliensis from the cultured promastigotes (Figure C), confirming the diagnosis.
Throughout treatment and follow-up, the patient attended as an outpatient and was able to continue his regular employment. His ulcer healed with a typical paper-thin scar (Figure D). Such a scar is usually flat, atrophic and depigmented (similar to a burns scar), and persists lifelong.
Leishmania species are dimorphic protozoan parasites transmitted by female blood-sucking sandflies to mammalian hosts, in which they become obligate intra-macrophage parasites. More than 25 species of Leishmania are capable of producing disease in humans and animal reservoirs in tropical and subtropical regions.3 About 1.5 million new cases are documented each year in humans, and over 350 million people live in areas of active parasite transmission.4 Leishmaniasis is one of the top five diseases targeted by the World Health Organization Special Programme for Research and Training in Tropical Diseases (http://www.who.int/tdr/index.html).
Whether leishmaniasis appears in cutaneous, mucosal or visceral form is largely determined by the parasite species. The strength of the host’s cell-mediated immunity determines whether infection remains subclinical, is self-healing, or becomes disseminated, when death may result.4 Acquired cell-mediated immunity may be partially protective against reinfection and last long-term, but does not always prevent recurrences of infection or metastasis of parasites, particularly if immunity wanes, as happens in advanced HIV infection.
Leishmaniasis is categorised geographically as “New World” (Central and South America, and Texas in the United States) or “Old World” (the Mediterranean basin, the Middle East and Africa). Lutzomyia is the sandfly vector in the New World and Phlebotomus in the Old World.3 New World CL may present as an ulcer that remains localised (if caused by species such as L. mexicana) or may later disseminate (if caused by species such as L. amazonensis or L. braziliensis). L. braziliensis is the most prevalent in the rainforests of South America, where various forest mammals (including anteaters, sloths and possums) act as animal reservoirs for the disease.3 Infection with L. braziliensis results in slow-healing skin ulcers. These may be further complicated by mucosal disease, the incidence of which is about 3% in patients living in areas of endemicity.5 Mucosal leishmaniasis, which may appear from several months to decades after the initial infection, progressively destroys the oronasopharyngeal mucosa and underlying cartilaginous facial and upper airway structures.3-5 Without treatment, an affected person may die from secondary infection or airway compromise. Thus, treatment of cutaneous L. braziliensis infection is aimed at reducing the risk of progression to mucosal leishmaniasis.
There have been few controlled trials to determine the optimal management for L. braziliensis infection.5,6 Intravenous pentavalent antimony, which has traditionally been used to treat all forms of leishmaniasis since 1915, is associated with serious side effects, including cardiotoxicity and sudden death. Additionally, recent studies have shown it to have reduced efficacy for treatment of CL.6,7 Amphotericin preparations, which are more effective for treating visceral and mucosal leishmaniasis, also look promising for treating New World CL.7 However, amphotericin B is also frequently associated with toxicity, including infusion-related fever, nausea and chills (these may be reduced with paracetamol premedication); normochromic, normocytic anaemia; and reversible nephrotoxicity, manifest as elevated serum creatinine levels and electrolyte loss (these can be managed by prehydration with normal saline and by electrolyte supplementation). Delivering amphtotericin in liposomal form lowers the risk of nephrotoxicity, but there is less experience with this preparation in treating leishmaniasis and the cost is significantly greater than conventional amphotericin B.
Lessons from practice
Cutaneous leishmaniasis (CL) is increasingly seen in travellers and must be considered in the differential diagnosis of ulcers in travellers returning from regions of endemicity.
New World CL may be difficult to diagnose on histopathology alone, and attempts should be made to culture the organism to facilitate diagnosis.
Polymerase chain reaction analysis, now available in Australia, is necessary to speciate the Leishmania infection to direct appropriate management.
Amphotericin B is an effective alternative to more toxic traditional treatment with pentavalent antimony.
PCR is currently the method of choice for speciating all forms of leishmaniasis, as it has a high sensitivity and gives a species-specific diagnosis, facilitating specific treatment.1,2 To increase PCR sensitivity, the yield of diagnostic material can be enhanced by culturing biopsy specimens in vitro. In our RFLP analysis, the species-specific bands visualised in agarose gels allowed unequi-vocal differentiation of the isolate. Both assays targeted different loci and gave concurrent results. This is the first time, to our knowledge, that PCR speciation of Leishmania has been done in Australia. Previously, isolates have been sent for speciation to specialised laboratories overseas.
Although mucosal leishmaniasis is rarely reported in travellers returning from areas of endemicity, the incidence has increased as travel to Latin America has become more common. Observed cases of New World CL have doubled in The Netherlands and tripled in the United Kingdom in the past decade.8 Imported cases of leishmaniasis, including New World CL, have been reported in Australia.9,10
Australia and the Pacific region have long been considered free of endemic Leishmania species4 and suitable sandfly vectors, thus preventing locally acquired leishmaniasis. However, locally acquired CL has recently been reported in Australian kangaroos.11 Molecular analysis of the isolates confirmed the genus Leishmania, but was suggestive of a novel species. This finding raises questions about the vector, possible unrecognised human transmission in Australia, and even potential endemic establishment of imported Leishmania.
In conclusion, New World leishmaniasis is becoming more frequently reported among travellers, and the diagnosis must be considered in any traveller with a cutaneous ulcer who has come from an area of Leishmania endemicity. PCR speciation is necessary to optimise appropriate management of this potentially serious infection. Direct inoculation of tissue specimens into a Leishmania-specific culture medium may increase the yield of diagnostic material and thus enhance PCR sensitivity. Our case illustrates that amphotericin is an effective treatment for New World CL (although associated with reversible toxicity) and that an affected patient can be managed successfully as an outpatient under close supervision.
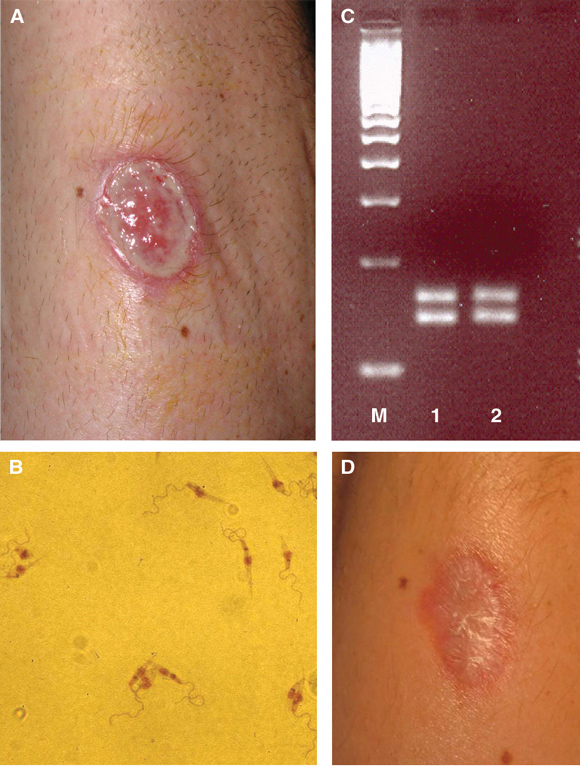
A: Non-healing New World cutaneous leishmaniasis ulcer on the left forearm. B: Metacyclic Leishmania promastigotes in culture medium, Day 7.The parasites have a characteristic coiled, highly motile flagellum at the apical end of an elongated body (1020 mm in length) containing a round nucleus and rod-shaped kinetoplast. C: Molecular banding pattern in agarose gel after PCR analysis. The banding pattern resulting from restriction fragment length polymorphism PCR analysis was consistent with Leishmania braziliensis DNA (M: a commercial 100-base-pair molecular marker [EZ Load 100 bp molecular ruler; Bio-Rad Laboratories, Hercules, Calif, USA]; 1: L. braziliensis control strain; 2: patient sample). D: Healed ulcer, 4 weeks after treatment with amphotericin B. | |||||||||||||||
- 1. Schonian GA, Nasereddin N, Dinse C, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis 2003; 47: 349-358.
- 2. Rotureau B, Ravel C, Couppie P, et al. Use of PCR-restriction fragment length polymorphism analysis to identify the main new world Leishmania species and analyse their taxonomic properties and polymorphism by application of the assay to clinical samples. J Clin Microbiol 2006; 44: 459-467.
- 3. Bryceson ADM. Leishmaniasis. In: Cook GC, editor. Manson’s tropical diseases. London: WB Saunders, 1996: 1213-1245.
- 4. Herwaldt BL. Leishmaniasis. Lancet 1999; 354: 1191-1199.
- 5. Jones T, Johnson WJ, Barretto A, et al. Epidemiology of American cutaneous leishmaniasis due to Leishmania braziliensis braziliensis. J Infect Dis 1987; 156: 73-83.
- 6. Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 2003; 16: 397-401.
- 7. Brown M, Noursadeghi M, Boyle J, Davidson RN. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol 2005; 153: 203-205.
- 8. Schwartz E, Hatz C, Blum J. New World cutaneous leishmaniasis in travellers. Lancet Infect Dis 2006; 6: 342-349.
- 9. Ju O, Grove DI, Jaksic WJ, Dart GW. Visceral leishmaniasis: a trip to the Greek Islands is not always idyllic. Med J Aust 2004; 181: 446-447. <MJA full text>
- 10. Maguire GP, Bastian I, Arianayagam S, et al. New World cutaneous leishmaniasis imported into Australia. Pathology 1998; 30: 73-76.
- 11. Rose K, Curtis J, Baldwin T, et al. Cutaneous leishmaniasis in red kangaroos: isolation and characterisation of the causative organisms. Int J Parasitol 2004; 34: 655-664.





We wish to acknowledge the Department of Microbiology, South Eastern Area Laboratory Services, St George Hospital, for their support in isolating and maintaining the Leishmania parasite. We would also like to thank Associate Professor John Harkness of St Vincent’s Hospital Microbiology Department for his support in setting up the molecular testing for Leishmania.
None identified.