Lowering serum lipid levels is a key component of preventive management in type 2 diabetes.1 Monitoring of lipid levels and prescription of lipid-lowering therapies are among the most common reasons for patients with diabetes to see their general practitioners.2 We used data from the NEFRON study (the National Evaluation of the Frequency of Renal Impairment cO-existing with NIDDM [Non-Insulin Dependent Diabetes Mellitus]) to describe how often patients with type 2 diabetes attain lipid treatment goals in Australian primary care and to examine factors that influence the management of dyslipidaemia.
The NEFRON study was designed as an incident-driven, cluster-stratified survey of patients with type 2 diabetes in Australian primary care.3 Sample selection and its representation of general practice are described in detail elsewhere.3 Five hundred investigators were enrolled in the study, representative of the regional distribution of GPs across Australia.3 The mean age (52 years) and number of sessions (43 per week) were also similar to those recorded for all registered GPs in Australia (50 years and 41 sessions per week, respectively). The study was approved by the Royal Australian College of General Practitioners (RACGP) National Research and Evaluation Ethics Committee. Data were collected between April and September 2005.
GPs were asked to complete a de-identified case report form for consecutive patients with type 2 diabetes, capturing clinical history, physical examination and laboratory results, including fasting serum lipid concentrations.3 No attempt was made to standardise results from different laboratories or regions, but rather to reflect the raw results on which GPs habitually base management.
for individuals with established coronary heart disease and a total cholesterol level > 4.0 mmol/L (after appropriate dietary interventions); and
in the absence of overt coronary heart disease, for individuals with a total cholesterol level ≥ 5.5 mmol/L and a high-density lipoprotein (HDL) cholesterol level < 1.0 mmol/L (after appropriate dietary interventions).
Lipid-lowering medication was prescribed for 63.9% of patients with type 2 diabetes in the NEFRON study (2487/3893) (Box 1). Most patients received a statin (61.3%; 2388/3893). Fewer received a fibrate (2.5%; n = 96) or a cholesterol absorption inhibitor (1.5%; n = 59).
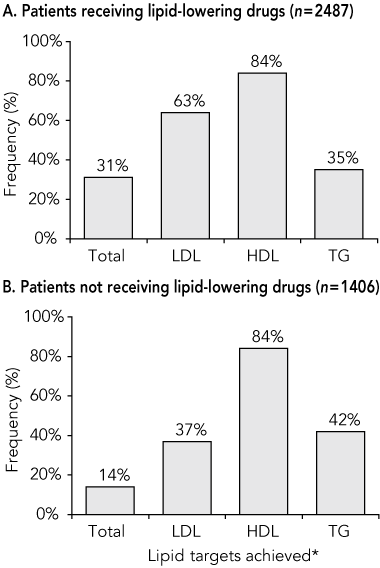
The National Heart Foundation target for total cholesterol (< 4.0 mmol/L) was achieved in 31% of patients receiving lipid-lowering therapy, while the LDL treatment target of < 2.5 mmol/L was achieved in 63% (Box 2A). Treated individuals achieving lipid targets were older, more likely to have an established history of cardiovascular disease (CVD), and also better glycaemic and blood pressure control (multivariate P < 0.001). Smoking, morbid obesity or the use of other medications that influence lipid levels (diuretics or β-blockers) did not affect the achievement of lipid targets. A third of treated patients (35%) achieved target levels for triglycerides (< 1.5 mmol/L), and 84% for HDL cholesterol (Box 2A).
About a third (36.1%) of patients with type 2 diabetes attending their GPs were not receiving any lipid-lowering medications (Box 1). A minority of these patients met optimal targets for lipid control (Box 2B). Many of these individuals had a high burden of cardiovascular risk markers or established CVD (Box 1). Despite this, only 11% of the patients not currently receiving lipid-lowering therapy met the criteria for government-subsidised treatment with a lipid-lowering agent current at the time of NEFRON (Box 3). Most of the untreated patients who did meet the criteria for subsidy had established CVD. Fewer than 4% of all patients without CVD and not receiving lipid-lowering medications met the criteria for subsidised primary therapy. This figure potentially reflected the small number of diabetic patients with HDL cholesterol level < 1.0 mmol/L (Box 2B), a required threshold for the subsidised prescription of lipid-lowering medication for primary prevention.
From the NEFRON data, we estimate that 82% of patients who were not receiving lipid-lowering therapy will now have access to subsidised therapy. Treatment of all these patients, as suggested by the low rates of untreated patients meeting previous subsidy criteria, would result in an increase of about 28% in lipid-lowering therapy in patients with type 2 diabetes, to potentially include 93% of all diabetic patients seeing their GPs in primary care. However, many of these patients have a low estimated cardiovascular risk (Box 3), making this a potentially costly way to ensure coverage of high-risk patients.
The identification and management of dyslipidaemia is a key component of management in patients with type 2 diabetes.1 Australian medical practice has widely adopted this message, with 64% of patients in the NEFRON study receiving lipid-lowering medications. Nonetheless, many patients failed to meet recommended lipid targets (Box 2). In addition, many people with diabetes and high cardiovascular risk were not treated, possibly because of their failure to meet HDL cut-offs for subsidised prescription. These data have important implications for the appropriate stratification and management of patients with type 2 diabetes in Australia.
A principal aim of the NEFRON study was to identify key management issues during consultation between an individual with type 2 diabetes and their GP. As a clinic-based, incident-driven study it has a number of limitations, being inherently biased towards patients who regularly attend their GPs. This was a deliberate approach to estimate the characteristics of patients seen by GPs every day in Australia. However, bias of GPs against recruiting patients with complex conditions or those with whom they were not familiar cannot be excluded. While extrapolation to a wider community of patients with type 2 diabetes is inappropriate, these results are nonetheless consistent with population-based studies,5 where fewer than half of all patients with type 2 diabetes achieved lipid treatment goals when assessed on a single occasion.
Although every effort was made to ensure a representative distribution of practices,3 NEFRON investigators were selected from 1500 GPs who initially expressed interest in participating in the study. While NEFRON investigators had similar age, sessions per week and regional distribution to Australian GPs generally, selection bias in relation to participating investigators, their prescribing practice and subsequently enrolled diabetic patients limit the generalisability of these data.
Many patients receiving a lipid-lowering drug still failed to achieve treatment goals for total cholesterol and triglycerides (Box 2). It should be acknowledged that achieving these goals can be difficult, particularly in patients with obesity, elderly patients, and those with chronic kidney disease who make up the majority of Australians with type 2 diabetes. Previous studies have suggested that inadequate dosing may explain failure to achieve lipid targets.6,7 Other important barriers include patient compliance, particularly as many patients receive multiple medications (Box 1),8,9 are elderly,9 or have reduced ability to pay for medications. Studies have suggested that long-term adherence to prescribed lipid-lowering medications is only 30%, and that non-adherence to statin therapy is associated with a detectable excess of CVD.10 In the NEFRON study, no descriptive characteristics clearly predicted patients on lipid-lowering medication who did not achieve therapeutic targets. These were not serially recalcitrant patients, as fewer than half of treated patients not meeting targets could be identified from elevated HbA1c (glycated haemoglobin) or blood pressure levels.
In the NEFRON study, over half of those patients not meeting National Heart Foundation targets did not receive any lipid-lowering therapy. This discrepancy may be partly explained by the regulations at the time that limited the subsidy for lipid-lowering medications, in the absence of overt coronary heart disease, to diabetic individ-uals with total cholesterol > 5.5 mmol/L and HDL < 1.0 mmol/L. However, low HDL levels are uncommon in this population, as estimated by the non-standardised laboratory tests on which they base their routine assessment. The changes to PBS eligibility criteria for lipid-lowering drugs will substantially improve coverage in patients with diabetes, with almost all high-risk patients now having unrestricted access (Box 3). If, like those currently treated, nearly two thirds of these patients can also maintain their LDL cholesterol < 2.5 mmol/L, then the cost of these changes will be saved many times over.
1 Clinical characteristics of patients with type 2 diabetes from the NEFRON study, by use of lipid-lowering medications
3 Distribution of cardiovascular risk* in patients not receiving lipid-lowering therapy
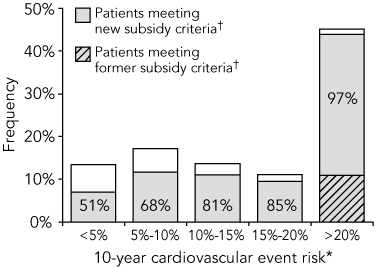
* Risk estimated by the UKPDS (United Kingdom Prospective Diabetes Study) calculator.4 † Criteria for subsidy of lipid-lowering therapy from the Australian Pharmaceutical Benefits Scheme. Percentage in shaded section represents the percentage of patients in this risk group who would meet the new criteria. |
Received 7 July 2006, accepted 23 October 2006
- Merlin C Thomas1
- Paul J Nestel2
- Baker Heart Research Institute, Melbourne, VIC.
This study was made possible with the generous help of GP investigators from around Australia. The study was supported by the Baker Heart Research Institute, Kidney Health Australia and Servier Australia. Data and project management services were provided by Quintiles Pty Ltd (Australia), and statistical services by Statistical Revelations Pty Ltd, Australia.
Merlin Thomas received an honorarium of $15 000 for his role in design, analysis and interpretation of the study, and writing of this paper.
The NEFRON study was conducted as a collaboration between the Baker Heart Research Institute, Kidney Health Australia and Servier Australia, which had equal roles in the study design, writing of this article and the subsequent decision to submit for publication. The study was unconditionally funded by Servier Australia.
- 1. Vijan S, Hayward RA. Pharmacologic lipid-lowering therapy in type 2 diabetes mellitus: background paper for the American College of Physicians. Ann Intern Med 2004; 140: 650-658.
- 2. Charles J, Ng A, Miller G. Management of type 2 diabetes in Australian general practice. Aust Fam Phys 2006; 35: 378-379.
- 3. Thomas MC, Weekes AJ, Broadley OJ, et al. The burden of chronic kidney disease in Australian patients with type 2 diabetes (the NEFRON study). Med J Aust 2006; 185: 140-144. <MJA full text>
- 4. Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond) 2001; 101: 671-679.
- 5. Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L-TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid-lowering therapy and achieving low-density lipoprotein cholesterol goals. Arch Intern Med 2000; 160: 459-467.
- 6. Putzer G, Roetzheim R, Ramirez AM, et al. Compliance with recommendations for lipid management among patients with type 2 diabetes in an academic family practice. J Am Board Fam Pract 2004; 17: 101-107.
- 7. Marcelino JJ, Feingold KR. Inadequate treatment with HMG-CoA reductase inhibitors by health care providers. Am J Med 1996; 100: 605-610.
- 8. Kulkarni SP, Alexander KP, Lytle B, et al. Long-term adherence with cardiovascular drug regimens. Am Heart J 2006; 151: 185-191.
- 9. Raffel OC, White HD. Drug insight: statin use in the elderly. Nat Clin Pract Cardiovasc Med 2006; 3: 318-328.
- 10. Blackburn DF, Dobson RT, Blackburn JL, Wilson TW. Cardiovascular morbidity associated with nonadherence to statin therapy. Pharmacotherapy 2005; 25: 1035-1043.





Abstract
Objective: To examine the frequency of dyslipidaemia and treatment with lipid-lowering drugs in patients with type 2 diabetes managed in Australian primary care.
Design, setting and participants: The NEFRON study (National Evaluation of the Frequency of Renal Impairment cO-existing with NIDDM [Non-Insulin Dependent Diabetes Mellitus]) was an incident-driven, cluster-stratified survey of 3893 patients with type 2 diabetes from across Australian primary care between April and September 2005.
Main outcome measures: The most recent fasting lipid levels were compared with therapeutic targets for lipid control and current prescribing guidelines.
Results: 64% of patients with type 2 diabetes presenting in primary care received lipid-lowering medication. Despite the widespread use of statins (61%), 75% of patients had a total cholesterol level ≥ 4.0 mmol/L, and 47% had a low-density lipoprotein (LDL) cholesterol level ≥ 2.5 mmol/L. Few untreated patients met the Australian Pharmaceutical Benefits Scheme (PBS) criteria current at the time for subsidised primary prevention with lipid-lowering agents (4%). However, new PBS subsidy criteria will potentially include 93% of all diabetic patients seeing their general practitioner in primary care.
Conclusion: Changes in the provision of subsidised therapy for high-risk diabetic patients are long overdue. However, more needs to be done to optimise management strategies, which still fail to achieve treatment targets in many treated patients.