Diabetes is the leading cause of end-stage kidney disease (ESKD) in Australia,1 and chronic kidney disease (CKD) in individuals with diabetes is associated with adverse outcomes, which contribute to significant morbidity and premature mortality.2-4 For example, an individual with diabetes and an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 has over 10 times the risk of cardiovascular disease compared with an individual with diabetes and normal kidney function.2 Consequently, the identification of CKD has significant implications for the routine management of patients with diabetes.
GPs were recruited on the basis of interest in participating in the study. Expressions of interest were invited via a mail-out conducted in February 2005 using a commercial database of all 18 810 registered GPs from all jurisdictions in Australia (Pharbase, Dendrite, Sydney, NSW). Expressions of interest were stratified according to location within each state (rural or urban) in a classification derived from Rural, Remote and Metropolitan Area categories.5 A number of expressions of interest from each stratum, proportional to the census population (Australian Bureau of Statistics, 2001), were then randomly selected by Quintiles Strategic Research Services (Melbourne, VIC) using SPSS version 13.0 (SPSS Inc, Chicago, Ill, USA), to make up a total of 500 GP investigators nationally (Box 1). In the event that a cohort did not achieve target levels of GP registration, another mail-out was targeted to the sector. In the event of investigator withdrawal from the study, the geographically closest GP who had returned an expression of interest but not been selected was approached as a potential replacement.
The sample size was selected on the basis of data from the AusDiab study that identified 28% of individuals with diabetes as having an eGFR < 60 mL/min/1.73 m2.6 As it is estimated that about 0.6 million Australians have known diabetes, a sample of 4238 patients was planned for NEFRON, to allow a 95% confidence interval of 26.5%–31.5%, around a true frequency of 28%. Five hundred GP investigators, each recruiting 10–15 consecutive patients, were predicted to generate this sample size, based on rates of investigator dropout and protocol violation in previous Australian research studies in primary care.7
GPs completed a case report form for each eligible patient, which captured demographic and clinical information (see Box 2). In addition, GPs recorded non-standardised results of routine physical examination (height, weight, waist circumference, blood pressure [seated, right arm, diastolic at Korotkoff phase V], resting pulse rate and rhythm) and data from the most recent blood tests and urinalysis, including serum creatinine, urea, electrolytes, glycated haemoglobin (HbA1c), fasting glucose and lipid levels, and urinary albumin and creatinine levels.
For our study, CKD was defined according to standard Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines: individuals with an eGFR < 60 mL/min/1.73 m2 or evidence of kidney damage on urinalysis (eg, albuminuria) were defined as having CKD.8 For primary analyses, the presence of an eGFR < 60 mL/min/1.73 m2 was determined as a categorical variable using the four-variable formula proposed by the Modification of Diet in Renal Disease (MDRD) Study, which forms the basis of automatic reporting of GFR in Australia.9 This has been shown to be a reliable tool for determining impaired kidney function in older individuals10 and Australians with type 2 diabetes,11 where serum creatinine measurements are calibrated with the MDRD methodology. Albuminuria was stratified according to International Diabetes Federation guidelines12,13 as follows:
Microalbuminuria — urinary albumin–creatinine ratio (ACR) of 3.5–35 mg/mmol (women) or 2.5–25 mg/mmol (men).
Macroalbuminuria — urinary ACR > 35 mg/mmol (women) or > 25 mg/mmol (men).
Normoalbuminuria — urinary ACR < 3.5 mg/mmol (women) or < 2.5 mg/mmol (men).
Patients with macro- or microalbuminuria were deemed to have an elevated urinary ACR.
Multivariate predictors of a reduced eGFR (defined as eGFR < 60 mL/min/1.73 m2) were identified using reverse stepwise logistic regression analysis and expressed as their odds ratios. Model validity was confirmed using the Akaike Information Criterion.14 The residuals from this model were checked, and no major departures from the assumptions of randomness and normality were seen. The influence of each patient on the model was also examined, and no individual patient had an undue impact on it. All regression diagnostics available in the SAS computer program (SAS Institute, Cary, NC, USA) were produced and plotted. In particular, the Pearson residuals and deviance residuals were used to identify observations that were not well explained by the model. Plots were obtained and examined for observations with unduly large residuals.
Expressions of interest were received from 1445 GPs across Australia. From the 500 GPs selected, 117 withdrew before submitting data; 86 of these were replaced by GPs from the same geographic region. Of the 469 GPs, 348 submitted data within the study timeframe. The distribution of GP investigators is shown in Box 1.
Informed consent was gained and data were collected from 3893 patients with type 2 diabetes. Their demographic and clinical details are summarised in Box 2. For the group as a whole, 52% were male, median age was 66 years, and median duration of diagnosed diabetes was 6 years. Most patients (82.5%) were of European ancestry, 10.2% were Asian, and 3.7% were identified as Indigenous Australians by their GPs. According to body mass index criteria,15 83.6% of patients were overweight or obese.
The frequency of CKD in patients with type 2 diabetes is shown in Box 3. Almost one in every four patients with type 2 diabetes attending their GPs had a reduced eGFR (< 60 mL/min/1.73 m2) (23.1%; 95% CI, 21.8%–24.5%), and more than one in three had an elevated urinary ACR (34.6%; 95% CI, 33.3%–35.9%). There was an overlap of 10.4% of patients who had both a reduced eGFR and an elevated urinary ACR. Thus, almost one in every two patients with type 2 diabetes seen by their GP had CKD, as defined by K/DOQI guidelines (47.1%; 95% CI, 45.8%–48.4%) (Box 3).16 There was no clinically significant influence of clustering in the estimated frequency of endpoints (data not shown).
The main predictors of a reduced eGFR are shown in Box 2. More women than men with type 2 diabetes had a reduced eGFR (adjusted odds ratio, 2.27; 95% CI, 1.75–2.95). These sex-specific differences were not explained by older age or longer duration of diabetes in women. Even in individuals with a short duration of disease (< 5 years), more women than men had a reduced eGFR.
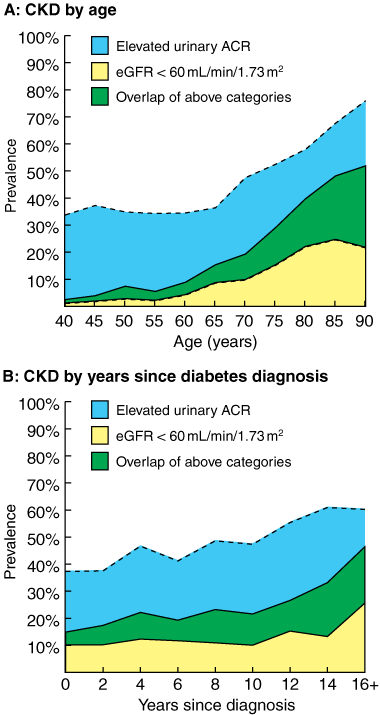
Individuals aged 65 years or over were also more likely to have a reduced eGFR, with more than a third (35.1%) in this age group having an eGFR < 60 mL/min/1.73 m2, compared with fewer than 9% of individuals aged less than 65 years (Box 4A). The frequency of a reduced eGFR also increased in proportion to the duration of diabetes (P = 0.02, Box 4B). However, it was also observed that 14.2% of all patients in whom type 2 diabetes had been diagnosed within the previous year had an eGFR < 60 mL/min/1.73 m2, increasing to 30.2% if patients were aged 65 years or over at the time of diagnosis.
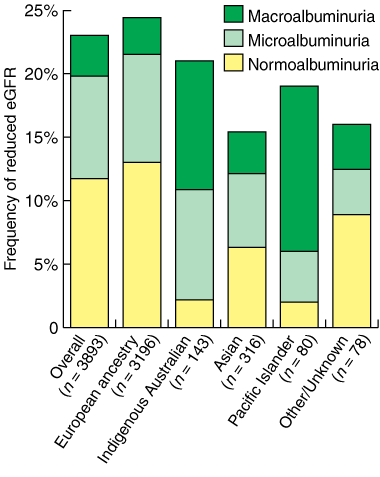
Unadjusted data showed a similar frequency of reduced eGFR across ethnic groups, with a significantly lower frequency only in Asian individuals (Box 5). However, this difference was eliminated by adjustment for differences in age, sex and duration of diabetes.
Other independent risk factors for a reduced eGFR included albuminuria, treatment of hypertension, height, a history of macrovascular disease or urinary tract infection, or a first-degree relative with CKD (Box 2). The overall predictive ability of the model was good, with a c-statistic of 0.792 (likelihood ratio test, χ2 = 673.9; P < 0.001).
The distribution of albuminuria in the patients is shown in Box 3: 27.3% of patients had microalbuminuria (95% CI, 25.9%–28.7%), and 7.3% had macroalbuminuria (95% CI, 5.9%–8.6%). The frequency of an elevated urinary ACR was statistically similar in men and women with type 2 diabetes (36.9% v 34.0%; P = 0.07). There was no association between age or duration of diabetes and the frequency of an elevated urinary ACR (Box 4).
Almost half (45.1%) of the individuals with macroalbuminuria had a reduced eGFR, compared with 28.2% of those with microalbuminuria, and 19.0% of those with a urinary ACR in the normal range. Two out of every three patients had a normal urinary ACR, including over half of all those with a reduced eGFR (Box 3). However, there were significant ethnic differences in this pattern. Notably, 90% of Indigenous Australians and over 80% of Pacific Islanders with a reduced eGFR had an abnormal ACR, mostly in the macroalbuminuric range. In contrast, only 46% of patients of European ancestry with a reduced eGFR also had an abnormal ACR, with fewer than 12% in the macroalbuminuric range (Box 5).
The estimation of GFR from a single measurement of serum creatinine concentration is also a potential source of imprecision. Nevertheless, the NEFRON data are consistent with findings from the small cohort of patients with known diabetes in the AusDiab study (n = 420), where 27.6% of individuals had an eGFR < 60 mL/min/1.73 m2 using the same estimation equation.6
In the NEFRON study, most patients with type 2 diabetes and an eGFR < 60 mL/min/1.73 m2 were older women. Some of this sex-related difference probably reflects variability in muscle mass and possibly protein intake that was not adequately modelled by the MDRD formula. The frequency of elevated urinary ACR was not significantly different between men and women with diabetes. Much of the increased frequency with age of an eGFR < 60 mL/min/1.73 m2 was attributable to an excess of older women with reduced eGFR but a urinary ACR in the normal range (Box 4). Similar findings have been reported in population studies6,17 and an Australian clinic study.18 While the nature of kidney damage in these patients is yet to be established, even in this population, an eGFR < 60 mL/min/1.73 m2 remains a potent independent risk marker that is clearly correlated with adverse outcomes.19
Of individuals with type 2 diabetes consulting their GPs, 29.5% had an abnormal ACR. Although these patients are broadly classified as having CKD,16 not all will have diabetic kidney disease, and a number of non-kidney diseases may contribute to an abnormal ACR (eg, hypertension, macrovascular disease, and heart failure). Nonetheless, regardless of aetiology, the presence and severity of albuminuria is strongly associated with adverse outcomes in this population.
The NEFRON study also highlights key racial and ethnic differences in the frequency of CKD. It is well known that Indigenous Australians have high rates of ESKD caused by diabetes.20 However, in the NEFRON study, the unadjusted frequency of CKD was similar in Indigenous and non-Indigenous populations. While this finding is clearly influenced by survival and selection biases inherent in this survey, it is consistent with results of surveys in other minority populations21 and the global DEMAND study.22 Secondly, although eGFR is well validated for adult white people, caution is required when applying eGFR to other patient groups, such as Indigenous Australians and people of Asian origin. Nonetheless, high rates of ESKD are more likely a result of the accelerated natural history of kidney disease in Indigenous Australians. Notably, over half of Indigenous Australian patients with type 2 diabetes and an eGFR < 60 mL/min/1.73m2 had an ACR in the macroalbuminuric range, compared with fewer than 12% of patients of European ancestry. If this is the real situation in the Australian community, then it is hardly surprising that Indigenous patients now make up over 8% of all Australians receiving dialysis, despite only 2% of Australians identifying themselves as Indigenous.
A working group representing the peak bodies of Australian nephrology, pathology and biochemistry, plus Kidney Health Australia, has proposed that, whenever a serum creatinine measurement is requested through any pathology service in Australia, the eGFR should be reported when its value is < 60 mL/min/1.73 m2.9 According to the NEFRON study, this means that an eGFR value will be reported for almost one in every four individuals with type 2 diabetes consulting their GPs. The same proportion will have an elevated urinary ACR reported, without an eGFR sufficient to trigger reporting. This large, and currently unrecognised, health care burden has the potential to overwhelm clinical practice as the frequency of diabetes and the number of ageing Australians continue to increase. It is hoped that efforts to increase the recognition of CKD will result in a significant change to clinical practice and lead to improved care and survival of patients with type 2 diabetes.
2 Clinical characteristics of patients with type 2 diabetes enrolled in the NEFRON study, by estimated glomerular filtration rate (eGFR) (data show the mean [SEM])
3 Venn diagram showing the distribution of albuminuria and estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 among patients with type 2 diabetes*
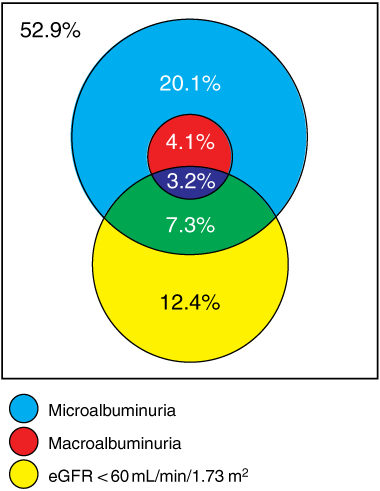
* The unshaded area denotes patients without chronic kidney disease (52.9%).
Received 27 February 2006, accepted 1 June 2006
- Merlin C Thomas1
- Andrew J Weekes2
- Olivia J Broadley2
- Mark E Cooper1
- Tim H Mathew3
- 1 Baker Heart Research Institute, Melbourne, VIC.
- 2 Servier Australia, Melbourne, VIC.
- 3 Kidney Health Australia, Adelaide SA.
This study was made possible with the generous help of 348 GP investigators from around Australia. Those submitting data for eight or more patients qualified for Royal Australian College of General Practitioners Continuing Professional Development points.
The study was conducted with the generous assistance of Servier Australia and could not have been completed without the meticulous work of Annie Solterbeck (Statistical Revelations, Melbourne).
Associate Professor Merlin Thomas is supported by a National Health and Medical Research Council–Diabetes Australia Research Trust Partnership Career Development Award.
The NEFRON study was conducted as a collaboration between the Baker Heart Research Institute, Kidney Health Australia and Servier Australia. It was unconditionally funded by Servier Australia.
Andrew Weekes and Olivia Broadley are employed by Servier Australia as Medical Affairs Manager and Associate Project Manager, respectively, and collaborated with the Baker Heart Research Institute and Kidney Health Australia in the study design and review of the submitted article.
Data collection and analysis were managed externally by Quintiles SRS and Statistical Revelations, Melbourne.
Data interpretation was primarily the responsibility of Merlin Thomas, who received an honorarium of $15 000 for his role in study design, analysis and interpretation, and writing of this article.
- 1. ANZDATA Registry. The twenty-sixth report. Adelaide: Australia and New Zealand Dialysis and Transplant Registry, 2003.
- 2. Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164: 659-663.
- 3. de Zeeuw D. Albuminuria, not only a cardiovascular/renal risk marker, but also a target for treatment? Kidney Int Suppl 2004; 92: S2-S6.
- 4. Stephenson JM, Kenny S, Stevens LK, et al. Proteinuria and mortality in diabetes: the WHO Multinational Study of Vascular Disease in Diabetes. Diabet Med 1995; 12: 149-155.
- 5. Australian Government Department of Primary Industries and Energy; Department of Human Services and Health. Rural, remote and metropolitan areas classification. 1991 census edition. Canberra: AGPS, 1994.
- 6. Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol 2003; 14 Suppl 2: S131-S138.
- 7. Welborn TA, Reid CM, Marriott G. Australian Diabetes Screening Study: impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Metabolism 1997; 46 (12 Suppl 1): 35-39.
- 8. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39 (2 Suppl 1): S1-S266.
- 9. Mathew TH. Chronic kidney disease and automatic reporting of estimated glomerular filtration rate: a position statement. Med J Aust 2005; 183: 138-141. <MJA full text>
- 10. Verhave JC, Fesler P, Ribstein J, et al. Estimation of renal function in subjects with normal serum creatinine levels: influence of age and body mass index. Am J Kidney Dis 2005; 46: 233-241.
- 11. Macisaac RJ, Tsalamandris C, Thomas MC, et al. Estimating glomerular filtration rate in diabetes: a comparison of cystatin-C- and creatinine-based methods. Diabetologia 2006; 49:1686-1689.
- 12. Atkins RC, Polkinghorne KR, Briganti EM, et al. Prevalence of albuminuria in Australia: the AusDiab Kidney Study. Kidney Int Suppl 2004; 92: S22-S24.
- 13. International Diabetes Federation Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels: IDF, 2005.
- 14. Agresti A. Categorical data analysis. 2nd ed. New York: John Wiley and Sons, 1990.
- 15. Cameron AJ, Welborn TA, Zimmet PZ, et al. Overweight and obesity in Australia: the 1999-2000 Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Med J Aust 2003; 178: 427-432. <MJA full text>
- 16. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139: 137-147.
- 17. Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 2003; 289: 3273-3277.
- 18. MacIsaac RJ, Tsalamandris C, Panagiotopoulos S, et al. Nonalbuminuric renal insufficiency in type 2 diabetes. Diabetes Care 2004; 27: 195-200.
- 19. Corsonello A, Pedone C, Corica F, et al. Concealed renal failure and adverse drug reactions in older patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci 2005; 60: 1147-1151.
- 20. Cass A, Cunningham J, Wang Z, Hoy W. Regional variation in the incidence of end-stage renal disease in Indigenous Australians. Med J Aust 2001; 175: 24-27. <MJA full text>
- 21. Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 2003; 41: 1-12.
- 22. Parving HH, Lewis JB, Ravid M, et al. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 2006; 69: 2057-2063.





Abstract
Objective: To estimate the frequency of chronic kidney disease (CKD) in a clinic-based sample of patients with type 2 diabetes in the setting of Australian primary care.
Design, setting and participants: Expressions of interest were invited from all registered general practitioners in Australia: 500 GP investigators were randomly selected from each stratum (state and urban versus rural location), proportional to the census population, and asked to recruit and provide data for 10–15 consecutively presenting adults with type 2 diabetes between April and September 2005.
Main outcome measures: Estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2 and evidence of kidney damage on urinalysis (eg, microalbuminuria).
Results: 348 GP investigators submitted data for 3893 individuals with type 2 diabetes (52% men; median age, 66 years). Almost one in every four patients consulting their GPs had an eGFR < 60 mL/min/1.73 m2 (23.1%; 95% CI, 21.8%–24.5%). More than one in three had an elevated urinary albumin–creatinine ratio (ACR) (34.6%; 95% CI, 33.3%–35.9%). There was an overlap of 10.4% of patients with both an eGFR < 60 mL/min/1.73 m2 and an elevated urinary ACR, meaning that almost one in two patients with type 2 diabetes consulting their GPs (47.1%; 95% CI, 45.8%–48.4%) had CKD. CKD was significantly more common in women, in older people, and in individuals with established macrovascular disease.
Conclusion: CKD is a common complication of type 2 diabetes, found in about half of all patients with type 2 diabetes consulting their GPs. Efforts to increase the recognition of CKD will lead to improved care, and possibly survival, of patients with type 2 diabetes.