Over a 3-year period, seven patients who were taking HMG-CoA reductase inhibitors (statins) presented to our respiratory service with interstitial pneumonitis. Clinical course varied, with the condition responding to prednisolone treatment and cessation of statins in three patients, and progressing slowly despite this management in another three, while one patient died of associated cardiac disease. While a causative role cannot be confirmed, clinicians should be aware of the possible association.
Two patients who presented with interstitial lung disease to our hospital’s respiratory service in 2000 prompted us to research a possible association between this disease and therapy with statins (hydroxymethylglutaryl-coenzyme A [HMG-CoA] reductase inhibitors). A literature review revealed several isolated cases of pneumonitis in the setting of statin therapy.1-3 Thereafter, we prospectively recorded patients referred to our unit who had interstitial lung disease and were undergoing statin therapy, where no other clear cause of the pneumonitis was evident. All cases have been reported to the Adverse Drug Reactions Advisory Committee.
Between January 2000 and December 2003, our service saw 58 new presentations of interstitial lung disease, including the two patients discussed above. Data on these patients were retrieved from an ambulatory care database of newly presenting patients kept prospectively by our service. Their diagnoses are shown in Box 1. Eight cases were thought to be drug-associated: five of these were potentially linked to statin therapy, two to nitrofurantoin and one to amiodarone. Another two patients were referred to our service during hospital admissions in other specialties not included in our database. Details of these patients were retained from consultation records.
Clinical details of the seven patients taking statin therapy are shown in Box 2. Most patients presented with dyspnoea and non-specific examination findings consistent with interstitial lung disease, such as bilateral crepitations on chest auscultation. None had clubbing. Some patients had a background of smoking and mild chronic obstructive airways disease, while others had no specific risk factors. Statins potentially implicated were atorva-statin (10–40 mg daily), pravastatin (40 mg daily) and simvastatin (10–40 mg daily). No patients were taking other medications known to be implicated in interstitial lung disease.
Pneumonitis was diagnosed based on clinical assessment, along with demonstration of interstitial infiltrates on high-resolution computed tomography and reduced transfer factor for carbon monoxide diffusion on lung function testing (Box 3).
Statins are the most often prescribed class of medication for treating hypercholesterolaemia. They act primarily by inhibiting HMG-CoA reductase, thereby inhibiting cholesterol biosynthesis and improving lipid profiles. However, recent research has revealed multiple immunomodulatory, vascular endothelial, antioxidant and other effects of statins.4 These so-called “pleiotrophic” effects have led to statins being studied in a host of unrelated clinical settings, including osteoporosis, multiple sclerosis and Alzheimer’s disease. While research is ongoing, it appears that statins have profound multisystem effects that extend well beyond lipid metabolism.
Statins are, on the whole, well tolerated, with the most commonly reported adverse effects being gastrointestinal upset, headache, rash and a dose-dependent elevation in serum levels of liver transaminases. The best characterised rare, but potentially serious, adverse effects are myopathy and polyneuropathy. These adverse effects are probably dose-related and may occur more often in patients taking medications known to inhibit statin metabolism.5
There are also a few reports of lupus-like syndromes, polymyositis/dermatomyositis with lung involvement, and hypersensitivity pneumonitis associated with statin therapy.1-3,6-9 The timing of onset appears unpredictable, with many patients having been taking statin therapy for many months or years before symptoms develop. Clinical features varied in severity from mild dry cough and rash through to severe and progressive respiratory failure. Low-titre antinuclear antibody (ANA) positivity and a raised erythrocyte sedimentation rate were also described in some patients. There are only four reports of open lung biopsy in these cases, two showing hypersensitivity pneumonitis with granuloma formation, one showing diffuse alveolar damage and the other showing non-specific interstitial pneumonitis. Most — but not all — patients responded to drug cessation and therapy with prednisolone or other potent immunosuppressive agents.2,8,9
One previous case of pneumonitis has been reported in the setting of statin therapy, which was confirmed by open lung biopsy, and where the findings closely resembled those in amphiphilic drug toxicity, such as that reported with amiodarone. The authors hypothesised that a toxic mechanism, possibly mediated by statin effects on lipid metabolism, led to the observed intralysosomal lamellar inclusions in pneumocytes and interstitial cells.9 Thus, while not fully characterised, there is a putative mechanism through which statins may cause interstitial pneumonitis.
2 Clinical details of seven patients with interstitial lung disease receiving statin (HMG-CoA reductase inhibitor) therapy
- 1. Hill C, Zeitz C, Kirkham B. Dermatomyositis with lung involvement in a patient treated with simvastatin. Aust N Z J Med 1995; 25: 745-746.
- 2. Liebhaber M, Wright R, Gelberg H, et al. Polymyalgia, hypersensitivity pneumonitis and other reactions in patients receiving HMG-CoA reductase inhibitors: a report of ten cases. Chest 1999; 115: 886-889.
- 3. De Groot RE, Willems LN, Dijkman JH. Interstitial lung disease with pleural effusion caused by simvastin. J Intern Med 1996; 239: 361-363.
- 4. Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21: 1712-1719.
- 5. Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation 2004; 109 (23 Suppl 1): III50-III57.
- 6. Ahmad S. Lovastatin-induced lupus erythematosus. Arch Intern Med 1991; 151: 1667-1668.
- 7. Bannwarth B, Miremont G, Papapietro PM. Lupuslike syndrome associated with simvastatin. Arch Intern Med 1992; 152: 1093.
- 8. Fauchais AL, Iba Ba J, Maurage P, et al. [Polymyositis induced or associated with lipid-lowering drugs: five cases] [French]. Rev Med Interne 2004; 25: 294-298.
- 9. Lantuejoul S, Brambilla E, Brambilla C, Devouassoux G. Statin-induced fibrotic nonspecific interstitial pneumonia. Eur Respir J 2002; 19: 577-580.





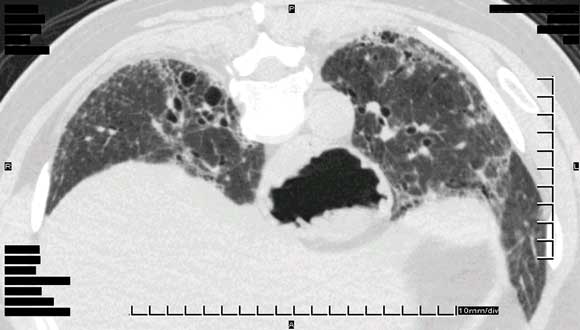
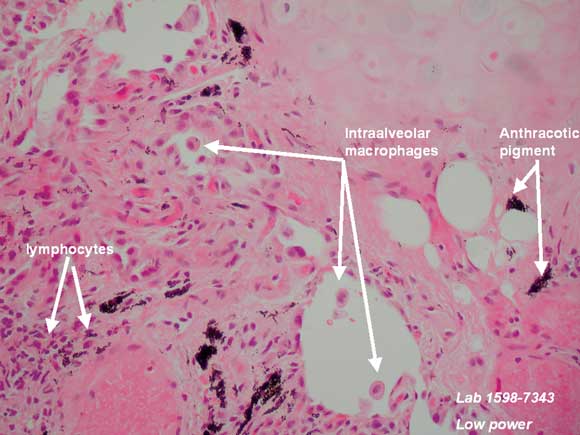
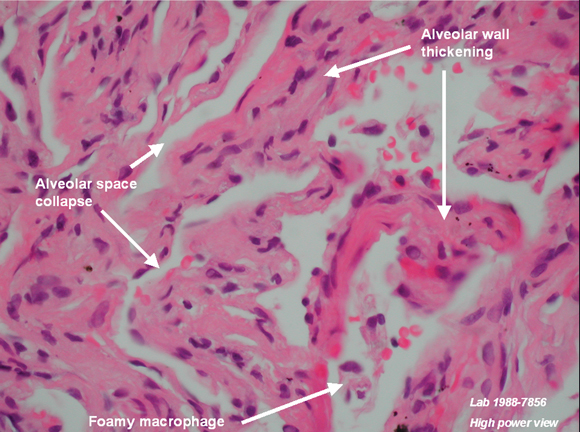
We thank Dr R Spokes and Dr A Jones, Pathologists, and Dr C Hair, Pathology Registrar, Pathcare Consulting Laboratories, for preparing and annotating histological slides, and Dr D Lun, Radiologist, Barwon Medical Imaging, for providing the high-resolution computed tomography image.
None identified.