In Australia, 26 111 patients received coronary stents for the treatment of coronary artery stenosis over the 12-month period from July 2002 to June 2003.1 The major drawback of the procedure is in-stent restenosis, with rates of 10%–60% reported using bare-metal stents (BMSs).2 Patients at highest risk of restenosis include those with diabetes, long lesions or small vessel diameter.2
Recent meta-analyses of randomised controlled trials have provided strong evidence that drug-eluting stents (DESs) reduce the risk of restenosis.3,4 However, they are at least three times more costly than BMSs and have not been shown to reduce patient mortality or myocardial infarction rates.3-5
Interpretation of this evidence has led to different recommendations for DES use. In the United States, the Food and Drug Administration has restricted DES approval to “on label” use, that is, to patient groups sharing the same low-risk characteristics as those recruited in trials.6 In contrast, in the United Kingdom it has been suggested that DESs should be targeted to patients at high risk of restenosis, which in some cases may include “off label” use.4
At an additional up-front market cost of about A$40 million a year, and with an annual growth in procedure rates of over 10%, the adoption of DESs has significant resource implications for Australia. Our study was part of a review of DESs conducted by the Medical Services Advisory Committee to assist with decisions about public funding.7 To date, about 95% of stents inserted in privately insured patients have been DESs,7 but their use in public hospital patients has largely been restricted (except in Western Australia) to those considered at high risk of stent restenosis. The pressing questions are: what are the costs and benefits of DESs in an Australian setting, and will targeting DESs to specific patient subgroups maximise these benefits while limiting overall cost?
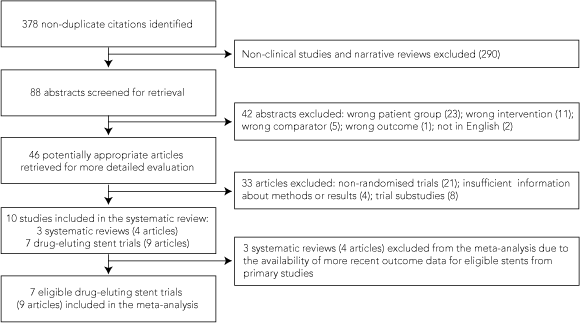
The electronic databases MEDLINE, Pre-Medline, EMBASE, Current Contents, CINAHL and the Cochrane Library database were searched to identify relevant studies published in English between January 1966 and June 2004 using Medical Subject Heading terms and text words for DES. Websites of health technology assessment agencies and bibliographies of relevant articles were also screened. All randomised controlled trials that evaluated clinical outcomes for the types of DES currently available in Australia (polymer-based sirolimus- or paclitaxel-eluting stents) were included (see Box 1 for eligibility criteria). Two independent reviewers achieved 100% agreement on the eligibility of articles identified by our search.
Four articles reporting on three systematic reviews and nine reporting on seven randomised controlled trials were appraised (Box 2). The systematic reviews included trials of ineligible stent types (not in current use in Australia), and in some cases did not include the most recent published data from eligible trials. Our review is based on published data from the remaining seven eligible trials.
Two independent reviewers assessed study quality according to the National Health and Medical Research Council’s quality checklist.9
Data about the baseline characteristics of the study population and co-therapy protocols were extracted to assess variation between trials and determine the applicability of the results to Australian practice.
Binary event data for five clinical outcomes were extracted for the primary meta-analyses of stent safety and effectiveness at 12 months: in-stent thrombosis rates at the latest reported time point, mortality, acute myocardial infarction, coronary artery bypass grafting, and revascularisation of the stented lesion (referred to as “target-lesion revascularisation” [TLR]). Two trials reported outcomes up to 9 months and contributed data about stent thrombosis only.10,11
Of the five trials reporting 12-month outcomes, four reported on all-cause mortality12-15 and one reported on deaths from cardiac disease only.16 Both classifications were included in the meta-analysis of mortality rates. Definitions of myocardial infarction also varied slightly across the trials.
Relative risks (RRs) and risk differences were calculated for all specified outcomes. Where possible, data were pooled, and fixed effects were calculated by the Mantel–Haenszel method using RevMan (Review Manager) version 4.2.7 (Cochrane Collaboration, Oxford, UK). Data from paclitaxel and sirolimus trials were analysed separately, owing to the different biological actions of these agents. All P values are two-sided. Cochran’s Q statistic was used to assess heterogeneity between trials.
Subgroup analyses were planned to determine whether the relative and absolute benefits of DESs compared with BMSs were different in patient groups known to have a high risk of in-stent restenosis with BMSs compared with other patients. Included trials reported data on three pre-specified high-risk subgroups for these analyses: patients with diabetes, patients with small-vessel lesions (≤ 2.5 mm diameter), and patients with long lesions requiring more than one stent (> 18 mm). Where event rates for subgroups were reported only as percentages, the number of events was calculated by referring to related substudies and conference presentations.
The incremental cost-effectiveness ratio (ICER) of DESs compared with BMSs per TLR avoided at 12 months was calculated using the formula:
|
ICER = | CostDES – CostBMS | ||||||||||||||
EffectivenessDES – EffectivenessBMS | |||||||||||||||
The costs of DESs and BMSs were calculated by applying Australian costs to resource utilisation estimates for each procedure from the TAXUS IV15 and SIRIUS13 trials. Resource utilisation was estimated for the study pro-cedure, the associated hospital stay and the 12-month period after discharge. Costs were based on the Department of Health and Ageing’s National Hospital Cost Data Collection database,17 the Medicare Benefits Schedule18 and the Pharmaceutical Benefits Schedule.19 The “base-case” analysis costed a DES at $2400 and a BMS at $850, with an estimated average number of stents per patient of 1.5.
In our economic analysis, the clinical effectiveness of DESs versus BMSs was calculated as the absolute risk reduction in TLR (absolute risk reduction = RR reduction × baseline risk). An exploratory analysis was conducted to estimate the quality-of-life changes associated with this clinical endpoint using published data.20 Utility (quality-of-life) weights of 0.77 for patients who experienced an event (defined as any repeat catheterisation) and 0.85 for patients who experienced no events were combined with TLR rates from the meta-analysis to calculate quality-adjusted-life-years (QALYs). As clinical practice may vary between countries and between trials and routine clinical settings, and the costs of DESs may vary over time, sensitivity analyses were conducted to explore the uncertainty surrounding the estimates of costs and effects used in the base-case analysis as follows:
TLR rates in both BMS and DES groups were reduced from 100% to 75% and to 50% of trial-reported rates on the basis of observational and trial evidence about the increase in revascularisation rates when resten-osis is detected by routine angiography rather than clinical presentation alone.21-23
The number of stents used per lesion was varied between one and two, on the basis of Australian data showing that 17% of patients receiving stents have more than one stent per vessel.1
The rates of percutaneous coronary interventions for non-target lesions and diagnostic catheterisations were reduced from 100% to 50% of trial-reported rates, based on expert opinion about potential differences between trial protocols and routine clinical practice.
The cost of a DES was varied from $2400 up to $3700, based on information about varying stent prices between states and between public and private hospitals from a small survey conducted in August 2004 (unpublished data), and down to $2000, based on the possibility of a fall in market prices.
Utility weights were varied to 0.80 for patients who required a repeat revascularisation and to 0.86 for patients who required no repeat revascularisation, based on the results of the Stent-PAMI trial24 in patients with acute myocardial infarction who received angioplasty or BMSs.
Further details about the methods of economic evaluation are available in the full report.7
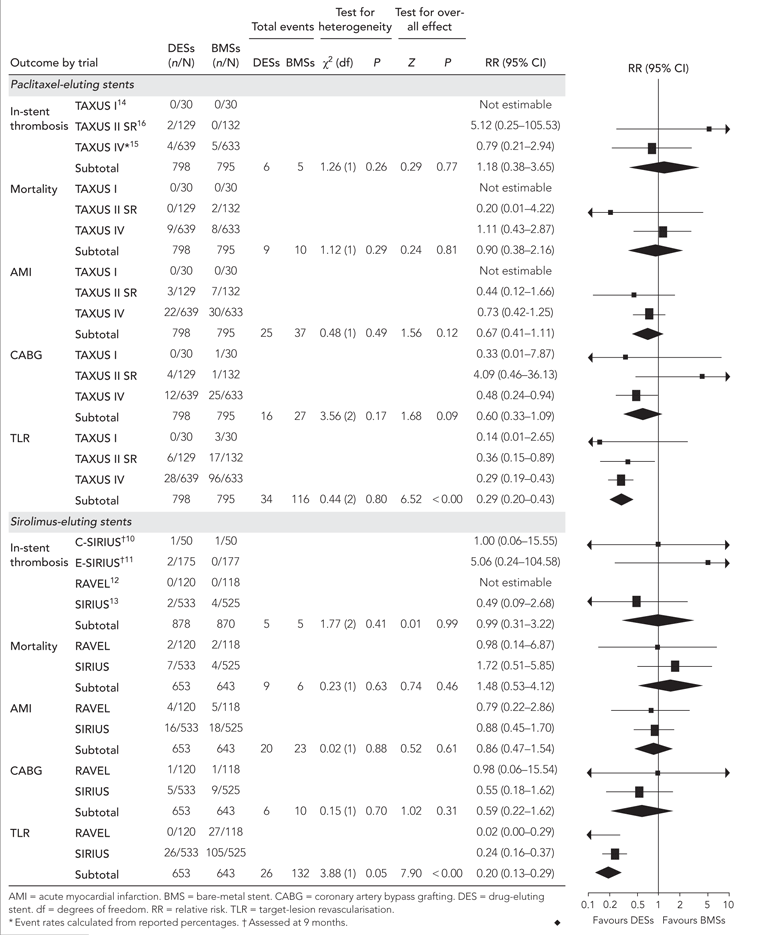
Four sirolimus-eluting stent trials (n = 1748 patients)10-13 and three paclitaxel-eluting stent trials (n = 1642 patients)14-16 were eligible for our review (Box 3).
Trials were designed to detect a difference in clinical13-15 or angiographic10-12,16 outcomes (Box 4). None were designed to detect a difference in survival rates.
All trials included patients with single de-novo coronary lesions of 51%–99% vessel diameter stenosis. TAXUS I14 also included patients with restenotic lesions. All trials excluded patients with acute myocardial infarction. Variation in the patient eligibility criteria and study procedures between trials are shown in Box 4. Although the patient and lesion characteristics at baseline were similar between treatment groups within trials, there was some variation between trials — in particular, between trials evaluating different stent types (Box 3).
All trials satisfied pre-specified criteria for high quality. All conducted an intention-to-treat analysis and reported at least 97% follow-up for clinical outcomes.
Stent thrombosis was reported in 0.6% of all trial participants, with no statistically significant difference between DES and BMS groups (Box 5). Among the 2889 trial participants for whom clinical outcomes were reported at 12 months, 34 deaths, 105 myocardial infarctions and 59 coronary artery bypass grafts were reported. Meta-analyses did not show a statistically significant difference in the relative risk of these events at 12 months between patients receiving paclitaxel- or sirolimus-eluting stents and those receiving BMSs (Box 5).
There were no statistically significant differences in the individual trial estimates for these outcomes, although moderate non-significant variation in the relative risk of coronary artery bypass grafting for paclitaxel-eluting stents versus BMSs was observed (Q statistic, 3.56; P = 0.17), with TAXUS IV15 reporting a statistically significant advantage for DESs versus BMSs.
Patients receiving DESs experienced significantly fewer TLRs at 12 months compared with those receiving BMSs. (Box 5).
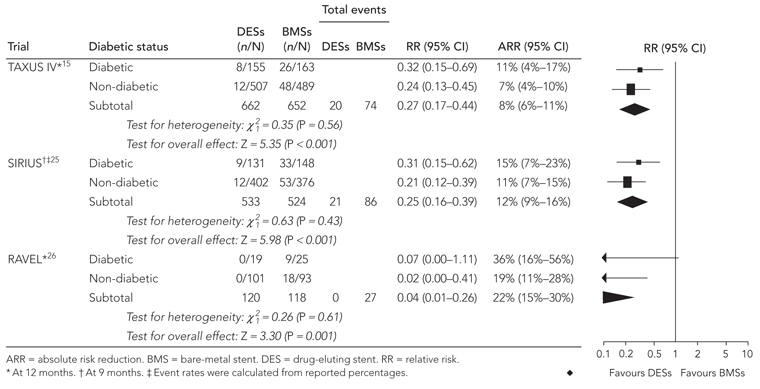
Three trials reported event rates for diabetic and non-diabetic subgroups at 9 months25 or 12 months.15,26 Each trial reported a statistically significant reduction in the absolute and relative risk of TLR for DESs compared with BMSs for patients both with and without diabetes (Box 6). Patient numbers were not large enough to statistically assess the differences in the absolute or relative benefits of DESs versus BMSs between diabetic and non-diabetic patients.
The TAXUS IV trial15 also reported a statistically significant relative risk reduction in TLR at 12 months for DESs compared with BMSs in patients with long lesions (> 20 mm) (RR, 0.23; P = 0.001) and those with a small target-vessel diameter (≤ 2.5 mm) (RR, 0.24; P < 0.0001). SIRIUS27 reported consistent results for subgroups of patients with long lesions (> 13.5 mm) and small vessel diameter (< 2.75 mm) that did not meet our prespecified definitions for high-risk lesions.
Estimates of the incremental cost per TLR avoided at 12 months ranged from $3746 (sirolimus trials) to $6117 (paclitaxel trials) (Box 7). Full details of this analysis are available in the Medical Services Advisory Committee report.7 Estimates of the incremental cost per additional QALY gained at 12 months ranged from $46 829 (sirolimus trials) to $76 467 (paclitaxel trials) (Box 7). Differences between these estimates are likely to reflect differences in the trial populations and trial methods, as well as possible differences in effectiveness between the different stent types.
These estimates are sensitive to the size of the clinical benefit associated with the DES, the number of stents used per patient, the cost of DESs, and the magnitude of quality-of-life benefit gained from avoiding revascularisation procedures. Results of the one-way sensitivity analyses indicated that, compared with BMSs, adopting DESs varied between being cost-saving to costing an extra $25 150 per TLR avoided at 12 months and an extra $314 385 per additional QALY gained at 12 months if trial-reported rates overestimate “true” TLR rates and costs by 50% (Box 8).
Varying the cost of DESs over the range $2000 to $3700, the cost per TLR avoided ranged from $120 to $24 993, while the cost per QALY gained ranged from $1504 to $312 418 (Box 8).
This is the first study to estimate the costs and benefits of DESs in current use in Australia. It provides strong evidence that DESs reduce the risk of revascularisation at 12 months by 71%–80% compared with BMSs in patients with single de-novo atheroscler-otic lesions (P < 0.0001).
Based on these data, and under base-case assumptions, the incremental cost of using DESs rather than BMSs was estimated at $A3750–$6100 per TLR avoided at 12 months, corresponding to $46 829–$76 467 per additional QALY gained.
There are considerable uncertainties surrounding these results. Firstly, the 12-month horizon of the published trial data may be insufficient to detect the true effects of treatment, including the possibility of late stent thrombosis.28 (However, unpublished longer-term trial data suggest that the safety and effectiveness of DESs are maintained at 2 years.)29
Secondly, despite the high quality of existing trials, the applicability of the results to Australian patients is unknown. All trials conducted routine angiography at 6–9 months after the procedure to assess in-stent restenosis. Applicability may be limited if a proportion of the TLRs reported in the trials resulted from angiographic findings that would otherwise have gone undetected in normal clinical practice, where angiography is reserved for patients with recurrent symptoms. Only three trials specified that revascularisation events must be clinically driven,10,13,15 and two of these trials reported that asymptomatic patients with ≥ 70% vessel diameter stenosis by quantitative coronary angiography were included in the definition of “clinically driven”.10,13 This problem applies both to patients receiving BMSs and those receiving DESs, and thus will not bias estimates of RR reduction; however, it may inflate estimates of absolute risk reduction, which are used to estimate cost-effectiveness.
In the worst-case scenario, the cost of DESs may reach $25 000 per TLR avoided and $314 385 per additional QALY gained. These figures are based on the assumption that trial rates of TLR overestimate rates in routine practice by 50%, as has been suggested by studies comparing angiographic findings of restenosis with symptoms of angina.21-23 The cost-effectiveness of DESs would be even less favourable if the true BMS revascularisation rate in Australian practice was as low as 5% (24%–34% of trial-reported rates), as suggested by one recent study.30 Moreover, the cost-effectiveness of DES would be further reduced if over 50% of patients received more than one stent per lesion. In Australia, 30% of patients receiving stents between July 2002 and June 2003 had more than one stent inserted, and 17% received the stents into a single coronary artery.1 On the other hand, the cost-effectiveness of DESs is highly dependent on their market price, falling to less than $4000 per QALY gained with a DES cost of $2000 and being potentially cost-saving at lower prices.
Finally, current evidence about the quality-of-life benefits of avoiding TLR has been derived from registry data of Canadian patients undergoing repeat catheterisation after a BMS insertion20 and a trial of acute myocardial infarction patients receiving balloon angioplasty versus BMS.24 This evidence may not apply to patients receiving stents in Australia. Our analysis assumes that the difference in quality of life at 12 months for patients receiving stents who experience repeat catheterisation events, with or without a revascularisation procedure, and those who do not can be entirely attributed to these events and is equivalent to that of avoiding TLR. Recent data suggesting inter-country differences in the valuation of health states indicate that, ideally, quality-of-life data specific to Australian patients should be sought.31
Current policies to restrict DES access to patient subgroups shown to have a high risk of TLR with BMSs are based on the plausible assumption that DESs offer a similar RR reduction to all patients — even those specifically excluded from the trials. If this is true, patients at the highest risk would obtain the maximum clinical benefit and, consequently, the most favourable cost-effectiveness ratio. No trials have been designed to investigate this hypothesis, although trial evidence does confirm that DESs are just as safe as BMSs and more effective in reducing TLR rates at 12 months in patients with diabetes and those with long lesions or small vessel diameters.
Unfortunately, there are insufficient subgroup data to establish whether such groups are likely to have a greater absolute benefit. Reports from sirolimus trials recruiting low-risk12 and higher-risk13 populations suggest that the treatment effect of DESs may not be equal across different subgroups of patients. There is also less evidence about the safety of DESs for these subgroups. Therefore, current restrictive policies need to be reassessed when additional evidence is available on such benefits and risks.
A recent article in the Journal called for the establishment of Australian registries to evaluate DESs.32 These registries could provide information on local patterns of use, patient risk, service costs and clinical outcomes. This would be valuable in determining whether Australian rates of revascularisation are consistent with the trial evidence.
However, registries are limited in their ability to compare DESs and BMSs directly. Substudies of existing trials, new trials recruiting more clinically-complex patients and meta-analyses using individual patient data would be valuable in determining the relative effectiveness of DESs compared with BMSs in patients with different baseline risks.
Ideally, funding decisions should be based on a direct comparison of competing interventions, with an acceptable cost per life-year saved or QALY gained. Unfortunately, there is a lack of such information for other cardiac interventions in Australia. An analysis of decisions for public funding under the Pharmaceutical Benefits Schedule between 1991 and 1996 indicates that an explicit decision threshold for public subsidy does not exist.33
1 Inclusion criteria for our study*
Study design
Systematic review or randomised controlled trial
Study population
Patients with a de-novo atherosclerotic lesion of the coronary artery, with or without inclusion of subgroups of patients with:
diabetes mellitus with single-vessel disease
stent restenosis
long lesions (> 18 mm)
small-diameter vessels (≤ 2.5 mm)
Intervention
Drug-eluting stents in current use in Australia:
polymer-based paclitaxel- or sirolimus-eluting stents
Comparator
Bare-metal stents
Outcomes at 12 months (one or more of the following)
mortality
myocardial infarction
coronary artery bypass grafting
revascularisation of the stented lesion (target-lesion revascularisation)
in-stent thrombosis at the latest reported time point
Publication type
English language
Reporting of study methods and results sufficient for quality assessment (excludes study abstracts)
* All criteria had to be satisfied for eligibility.
3 Patient and lesion characteristics reported by individual trials
Patient characteristics* |
Lesion characteristics† |
||||||||||||||
Trial |
n |
Mean age (years) |
Male |
Smoker |
Diabetes mellitus |
Hyper-tension |
Hyper- lipidaemia |
Unstable angina |
Prior MI |
Multi-vessel disease |
Lesion length (mm) |
Reference vessel diameter‡ (mm) |
|||
Paclitaxel trials (n = 1642) |
|||||||||||||||
TAXUS I14 |
61 |
64.9 |
89 |
51 |
18 |
64 |
81 |
32§ |
28 |
NR |
11 (4) |
3.0 (0.5) |
|||
TAXUS II16 |
267¶ |
60.1 |
76 |
25 |
15 |
62 |
NR |
35 |
40 |
NR |
11 (4) |
2.8 (0.5) |
|||
TAXUS IV15 |
1314 |
62.4 |
72 |
22 |
24 |
70 |
65 |
34 |
30 |
NR |
13 (6) |
2.8 (0.5) |
|||
Sirolimus trials (n = 1748) |
|||||||||||||||
C-SIRIUS10 |
100 |
60.5 |
69 |
37 |
24 |
52 |
85 |
51 |
45 |
40 |
14 (6) |
2.6 (0.3) |
|||
E-SIRIUS11 |
352 |
62.3 |
71 |
33 |
23 |
64 |
74 |
33 |
42 |
36 |
15 (6) |
2.6 (0.4) |
|||
RAVEL12 |
238 |
60.7 |
76 |
30 |
19 |
61 |
40 |
50 |
36 |
NR |
10 (3) |
2.6 (0.5) |
|||
SIRIUS13 |
1058 |
62.3 |
71 |
20 |
26 |
68 |
74 |
53 |
31 |
42 |
14 (6) |
2.8 (0.5) |
|||
MI = myocardial infarction. NR = not reported. * All patient characteristics except mean age are expressed as percentages. † Expressed as mean (SD). ‡ The vessel with stenosis that will undergo stenting in the trial. § Grade III–IV angina using the Canadian Cardiovascular Society classification (inability or marked limitation of ordinary activity, or rest pain). ¶ Excludes 269 patients allocated to medium-release DESs (not available in Australia) and their controls, who were therefore not eligible for our review. |
|||||||||||||||
4 Trial size, setting, lesion eligibility and study procedures for trials of paclitaxel- and sirolimus-eluting stents
Trial size and setting |
Study procedures |
||||||||||||||
Trial |
n |
Setting |
Primary outcome* |
Lesion exclusions |
Insertion procedure |
Type and average use of additional stents per patient |
Co-therapies† |
||||||||
Paclitaxel trials (n = 1642) |
|||||||||||||||
TAXUS I14 |
61 |
3 sites; Germany; Oct 2000–Mar 2001 |
Composite: death, MI, TVR, stent thrombosis at 30 days |
Long lesion requiring > 1 × 15 mm stent |
Pre-dilation |
Additional non-study stents selected by investigator; average stents/patient NR |
Clopidogrel 6 months |
||||||||
TAXUS II16 |
267‡ |
38 sites; Europe, USA, Canada, South America, Australasia; Jun 2001–Jan 2002 |
% Stent volume obstruction at 6 months |
Long lesion requiring > 1 × 15 mm stent |
Pre-dilation |
2nd stent BMS or DES, according to treatment allocation; 3rd stent selected by investigator; average stents/patient NR |
Clopidogrel or ticlopidine ≥ 6 months; ± glycoprotein IIb/IIIa receptor blocker (overall use, 12%) |
||||||||
TAXUS IV15 |
1314 |
73 sites; USA; Mar–Jul 2002 |
TVR at 9 months |
Long lesion requiring > 1 × 32 mm stent; ostial lesion; left main lesion; bifurcation lesion; calcified, tortuous or angulated lesion; thrombus-containing lesion |
Pre-dilation |
BMS or DES; 1.8% patients received > 1 stent; average stents/patient = 1.1 |
Clopidogrel 6 months; ± glycoprotein IIb/IIIa receptor blocker |
||||||||
Sirolimus trials (n = 1748) |
|||||||||||||||
C-SIRIUS10 |
100 |
8 sites; Canada; Nov 2001–Apr 2002 |
In-stent minimum lumen diameter at 8 months |
Ostial lesion; bifurcation lesion with side-branch requiring stenting; calcified lesion; thrombus- containing lesion |
Pre-dilation or direct stenting |
BMS or DES, according to treatment allocation; 40% patients received > 1 stent; average stents/patient = 1.5 |
Clopidogrel 2 months; ± glycoprotein IIb/IIIa receptor blocker |
||||||||
E-SIRIUS11 |
352 |
35 sites; Europe; Aug 2001–Feb 2002 |
In-stent minimum lumen diameter at 8 months |
Ostial lesion; calcified lesion; thrombus-containing lesion; bifurcation lesion with side-branch requiring stenting |
Pre-dilation or direct stenting |
BMS or DES, according to treatment allocation; 48% patients received > 1 stent; average stents/patient NR |
Clopidogrel or ticlopidine 2 months; ± glycoprotein IIb/IIIa receptor blocker |
||||||||
RAVEL12 |
238 |
19 sites; Europe, Mexico, Brazil; Aug 2000–Aug 2001 |
In-stent late luminal loss at 6 months |
Long lesion requiring > 1 × 18 mm stent; ostial lesion; calcified lesion; thrombus-containing lesion |
Pre-dilation |
BMS or DES, according to treatment allocation; average stents/patient NR |
Clopidogrel or ticlopidine 2 months; ± glycoprotein IIb/IIIa receptor blocker (overall use, 10%) |
||||||||
SIRIUS13 |
1058 |
53 sites; USA, Canada; Feb 2001–Sep 2002 |
Target-vessel failure |
Ostial lesion; bifurcation lesion |
Pre-dilation |
BMS or DES, according to treatment allocation; 27.7% patients received > 1 stent; average stents/patient = 1.4 |
Clopidogrel 3 months; ± glycoprotein IIb/IIIa receptor blocker (overall use, 60%) |
||||||||
BMS = bare-metal stent. DES = drug-eluting stent. MI = myocardial infarction. NR = not reported. TVR = target-vessel revascularisation. * Primary outcome reported in publication or outcome used to estimate sample size. † All trials reported intravenous intraprocedural heparin and daily aspirin. The three trials reporting on percentage use of glycoprotein IIb/IIIa receptor blocker in each study arm noted no differences by treatment group.12,18 ‡ Excludes 269 patients allocated to medium-release DESs and their controls (not eligible for our review). |
|||||||||||||||
6 Relative risk and absolute risk reduction of target-lesion revascularisation: drug-eluting stents compared with bare-metal stents in patients with and without diabetes
7 Incremental cost-effectiveness ratio per target-lesion revascularisation (TLR) procedure avoided and per quality-adjusted-life-year (QALY) gained at 12 months
|
SESs versus BMSs |
PESs versus BMSs |
|||||||||||||
Parameter |
SES |
BMS |
Difference |
PES |
BMS |
Difference |
|||||||||
Total cost at 12 months* |
$10 959 |
$10 339 |
$620 |
$10 887 |
$10 255 |
$632 |
|||||||||
TLR rates at 12 months† |
26/653 |
132/643 |
16.5% |
34/798 |
116/795 |
10.3% |
|||||||||
Cost per TLR avoided at 12 months |
$3746 per TLR avoided |
$6117 per TLR avoided |
|||||||||||||
QALY estimates at 12 months‡ |
0.847 |
0.834 |
0.013 |
0.847 |
0.838 |
0.008 |
|||||||||
Cost per additional QALY gained at 12 months |
$46 829 per TLR avoided |
$76 467 per TLR avoided |
|||||||||||||
BMS = bare-metal stent. PES = paclitaxel-eluting stent. SES = sirolimus-eluting stent. * Australian dollar costs were based on resources reported in the TAXUS IV15 and SIRIUS13 trials. †TLR rates were based on the results of our meta-analysis. ‡Utility weight was 0.77 for patients with revascularisation and 0.85 for patients with no revascularisation.20 |
|||||||||||||||
8 Sensitivity analyses exploring the effects of changing estimates of clinical practice, costs and effects*
|
Incremental cost per TLR avoided |
Incremental cost per QALY gained |
|||||||||||||
Variable |
SES |
PES |
SES |
PES |
|||||||||||
Base-case analysis† |
$3746 |
$6117 |
$46 829 |
$76 467 |
|||||||||||
Average number of stents per patient† |
|||||||||||||||
One |
Cost-saving |
Cost-saving |
Cost-saving |
Cost-saving |
|||||||||||
Two |
$8415 |
$13 595 |
$105 185 |
$169 940 |
|||||||||||
Rates of TLR |
|||||||||||||||
Reduced to 75% of trial rates |
$7527 |
$12 339 |
$94 093 |
$154 236 |
|||||||||||
Reduced to 50% of trial rates |
$15 320 |
$25 151 |
$191 500 |
$314 385 |
|||||||||||
Rates of PCI for non-target lesions and diagnostic catheterisations |
|||||||||||||||
Reduced to 50% of trial rates |
$4014 |
$6547 |
$50 180 |
$81 835 |
|||||||||||
Cost per DES† |
|||||||||||||||
Increased to $3700 |
$15 531 |
$24 993 |
$194 135 |
$312 418 |
|||||||||||
Reduced to $2000 |
$120 |
$309 |
$1504 |
$3867 |
|||||||||||
Utility weight‡ for TLR events† |
$20 813 |
$33 985 |
|||||||||||||
DES = drug-eluting stent. PCI = percutaneous coronary intervention. PES = paclitaxel-eluting stent. QALY = quality-adjusted-life-year. SES = sirolimus-eluting stent. TLR = target-lesion revascularisation. * For example, if the estimated average number of 1.5 stents per patient is reduced to one stent per patient, it will be cost-saving (ie, less costly and more effective) to use DESs compared with BMSs. If this estimate is increased to an average of two stents per patient, the incremental cost per TLR avoided increases to $8415 for SESs and $13 595 for PESs compared with BMSs. † Base-case analysis: mean, 1.5 stents/patient; $2000 per DES; $855 per BMS; utility weight for TLR, 0.77; utility weight for no TLR, 0.85. ‡ Estimated from Stent-PAMI trial.24 |
|||||||||||||||
Received 6 May 2005, accepted 29 August 2005
- Sarah J Lord1
- Felicity Allen2
- Luke Marinovich3
- David C Burgess4
- Kirsten Howard5
- Richard King6
- John J Atherton7
- 1 National Health and Medical Research Council Clinical Trials Centre, University of Sydney, Camperdown, NSW.
- 2 School of Public Health, University of Sydney, NSW.
- 3 Southern Health, Monash Medical Centre, Clayton, VIC.
- 4 Royal Brisbane and Women’s Hospital, Herston, QLD.
We gratefully acknowledge Marcus Ilton, Brendon Kearney, Leo Mahar, Paul McNairy, Sheila Rimmer and Edward Stafford, members of the advisory panel to the Medical Services Advisory Committee’s drug-eluting stent assessment report,7 for their generous contributions to the planning and interpretation of the broader systematic review on which our article is based. We thank Rhana Pike for her expert assistance in the preparation of this manuscript.
Sarah Lord, Kirsten Howard, Felicity Allen and Luke Marinovich have received funding from the Medical Services Advisory Committee for the assessment of drug-eluting stents. John Atherton is a member and Richard King a former member of the Committee.
- 1. Australian Institute of Health and Welfare. National hospital morbidity database 2002–03. Canberra: AIHW, 2004. Available at: http://www.aihw.gov.au/cognos/cgibin/ppdscgi.exe?DC=Q&E=/AHS/procedures_0203 (accessed Mar 2005).
- 2. Hoffmann R, Mintz GS. Coronary in-stent restenosis — predictors, treatment and prevention. Eur Heart J 2000; 21: 1739-1749.
- 3. Babapulle MN, Joseph L, Belisle P, et al. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 2004; 364: 583-591.
- 4. Hill R, Bagust A, Bakhai A, et al. Coronary artery stents: a rapid systematic review and economic evaluation. Health Technol Assess 2004; 8(35): iii–iv, 1-242.
- 5. Katritsis DG, Karvouni E, Ioannidis JP. Meta-analysis comparing drug-eluting stents with bare-metal stents. Am J Cardiol 2005; 95: 640-643.
- 6. Letter of approval of premarketing application for the CYPHER sirolimus-eluting coronary stent. 24 April 2003. Center for Devices and Radiological Health, Food and Drug Administration, US Department of Health and Human Services. Available at: http://www.fda.gov/cdrh/PDF2/P020026A.pdf (accessed Sep 2005).
- 7. Medical Services Advisory Committee. Drug-eluting stents. Canberra: Australian Department of Health and Ageing, 2005. Available at: http://www7.health.gov.au/msac/pdfs/reports/msacref30.pdf (accessed Aug 2005).
- 8. Moher D, Cook DJ, Eastwood S, et al. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet 1999; 354: 1896-1900.
- 9. National Health and Medical Research Council. How to review the evidence: systematic identification and review of the scientific literature. Handbook series on preparing clinical practice guidelines. Canberra: NHMRC, 1999.
- 10. Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am Coll Cardiol 2004; 43: 1110-1115.
- 11. Schofer J, Schluter M, Gershlick A, et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet 2003; 362: 1093-1099.
- 12. Morice MC, Serruys PW, Sousa JE, et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 2002; 346: 1773-1780.
- 13. Holmes DR Jr, Leon MB, Moses JW, et al. Analysis of 1-year clinical outcomes in the SIRIUS trial: a randomized trial of a sirolimus-eluting stent versus a standard stent in patients at high risk for coronary restenosis. Circulation 2004; 109: 634-640.
- 14. Grube E, Silber S, Hauptmann KE, et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation 2003; 107: 38-42.
- 15. Stone GW, Ellis SG, Cox DA, et al; TAXUS-IV Investigators. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation 2004; 109: 1942-1947.
- 16. Colombo A, Drzewiecki J, Banning A, et al; TAXUS II Study Group. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation 2003; 108: 788-794.
- 17. Australian Department of Health and Ageing. National hospital cost data collection 2002-03. Canberra: Australian Government, 2004. Available at: http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-casemix-costing-costmain1.htm (accessed Sep 2005).
- 18. Australian Department of Health and Ageing. Medicare Benefits Schedule Book. Canberra: Australian Government, 2004.
- 19. Australian Department of Health and Ageing. Pharmaceutical Benefits Schedule. Canberra: Australian Government, 2004.
- 20. Shrive FM, Manns BJ, Galbraith PD, et al. APPROACH Investigators. Economic evaluation of sirolimus-eluting stents. CMAJ 2005; 172: 345-351.
- 21. Cutlip DE, Chauhan MS, Baim DS, et al. Clinical restenosis after coronary stenting: perspectives from multicenter clinical trials. J Am Coll Cardiol 2002; 40: 2082-2089.
- 22. Fischman DL, Leon MB, Baim DS, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med 1994; 331: 496–501.
- 23. Holmes DR Jr, Vlietstra RE, Smith HC, et al. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA registry of the National Heart, Lung, and Blood Institute. Am J Cardiol 1984; 53: 77C-81C.
- 24. Cohen DJ, Taira DA, Berezin R, et al. Cost-effectiveness of coronary stenting in acute myocardial infarction: results from the stent primary angioplasty in myocardial infarction (Stent-PAMI) trial. Circulation 2001; 104: 3039–3045.
- 25. Moussa I, Leon MB, Baim DS, et al. Impact of sirolimus-eluting stents on outcome in diabetic patients: a SIRIUS (SIRolImUS-coated Bx Velocity balloon-expandable stent in the treatment of patients with de novo coronary artery lesions) substudy. Circulation 2004; 109: 2273-2278.
- 26. Abizaid A, Costa MA, Blanchard D, et al. Sirolimus-eluting stents inhibit neointimal hyperplasia in diabetic patients. Insights from the RAVEL Trial. Eur Heart J 2004; 25: 107-112.
- 27. Moses J, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 2003; 349: 1315-1323.
- 28. McFadden EP, Stabile E, Regar E, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet 2004; 364: 1519-1521.
- 29. Russell ME; Boston Scientific Corporation. Durability of the polymer-based, paclitaxel-eluting TAXUS stent in key patient subsets: two-year results from the TAXUS-IV trial. American College of Cardiology Annual Scientific Session; 2005 Mar 6-9; Orlando, Fla, USA.
- 30. Ward M. Cost-benefit of drug eluting stents — time for a reality check. Heart Lung Circ 2005; 14: 74-77.
- 31. Johnson JA, Luo NP, Shaw JW, et al. Valuations of EQ-5D health states: are the United States and United Kingdom different? Med Care 2005; 43: 221-228.
- 32. Chew DP. Cost-effectiveness of drug-eluting stents: if only all things were equal [editorial]. Med J Aust 2005; 182: 376-377. <MJA full text>
- 33. George B, Harris A, Mitchell A. Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in Australia (1991 to 1996). Pharmacoeconomics 2001; 19: 1103-1109.





Abstract
Objectives: To compare the safety, effectiveness and cost-effectiveness of drug-eluting coronary stents used in Australia with bare-metal stents and determine whether the benefits are greater for high-risk subgroups.
Data sources: MEDLINE, Pre-Medline, EMBASE, Current Contents, CINAHL and the Cochrane Library database were searched to identify eligible randomised controlled trials and systematic reviews published in English between January 1966 and June 2004.
Study selection: Seven randomised controlled trials that assessed polymer-based paclitaxel- or sirolimus-eluting stents versus bare-metal stents in patients with coronary atherosclerosis and reported on stent thrombosis, mortality, myocardial infarction, coronary artery bypass grafting or target lesion revascularisation.
Data extraction: Two independent reviewers appraised eligible studies and extracted data. Relative risks (RRs) were calculated for each outcome and pooled using the Mantel–Haenszel method.
Data synthesis: Rates of stent thrombosis, mortality, myocardial infarction and bypass grafts did not differ by stent type. Drug-eluting stents (DESs) resulted in a 71%–80% lower risk of revascularisation at 12 months (RR 0.29 [95% CI, 0.20–0.43] for paclitaxel-eluting stents [n = 1593 patients]; RR 0.20 [95% CI, 0.13–0.29] for sirolimus-eluting stents [n = 1296 patients]). Similar benefits were seen in several high-risk subgroups of patients: those with diabetes, lesion length > 20 mm and target-vessel diameter ≤ 2.5 mm. The benefits of DESs in these high-risk groups over lower-risk groups were inconclusive because of low numbers. The cost per revascularisation avoided by using DESs was A$3750–$6100, with an estimated cost per quality-adjusted-life-year (QALY) gained of A$46 829–$76 467. In sensitivity analyses, estimates varied from DESs being cost-saving to costing an additional $314 385 per QALY gained.
Conclusions: DESs are effective in reducing revascularisation. Estimates of cost-effectiveness are very sensitive to changes in estimates of their true effects in clinical practice, market price and the number of stents used per patient. Decisions to limit DESs to only patients at the highest risk of restenosis may improve their cost-effectiveness but will need to be reassessed when evidence is available to compare absolute benefits between patient groups.