Late last year, the Australian Government announced that it would amend the National Health and Medical Research Council Act 1992 (Cwlth) to clarify and streamline the governance of the National Health and Medical Research Council (NHMRC).1 The announcement came within days of the publication of an editorial in the MJA,2 which was critical of many aspects of the NHMRC and its governance. While the MJA undoubtedly has some impact on Australia’s health, the changes announced represented the government’s response to the findings of two reviews of health and medical research. Nevertheless, many in government and the research community would have, perhaps grudgingly, accepted the thrust of the criticisms in the editorial.2 There was an assertion that although the NHMRC was “world-class” in ethics and was continuing to fund excellent research, it was perceived to be no longer the leader in what it was originally established to do: to foster medical and public health research and then turn research evidence into improving individual health and health care in general.3
Changes to the NHMRC Act flowed from the government’s acceptance of the recommendations on the governance of federal bodies by a committee chaired by John Uhrig,4 which called for changes to government agencies to clarify their responsibilities and ensure that they were appropriately responsive to the elected government. For the NHMRC, as for the Australian Research Council, the changes resulted in the Chief Executive Officer (CEO) being made directly responsible to the relevant Minister. The changes to the NHMRC were also broadly in line with the recommendations of the Investment Review of Health and Medical Research (chaired by Mr John Grant),5 established in 2003 to report on implementation of the 2000 Strategic Review of Health and Medical Research (the Wills review).6 The Grant review found that many changes had been successfully implemented, but the NHMRC’s structure was still impeding the organisation’s ability to achieve its mandate. The MJA editorial was less kind; it described the NHMRC as “arthritically conservative”.2
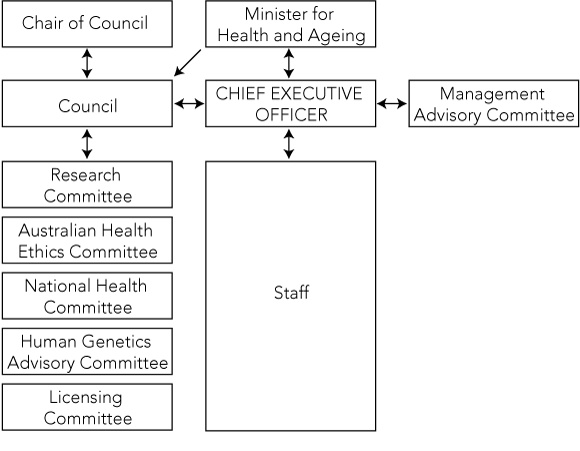
The revised National Health and Medical Research Council Act proclaimed on 1 July 20061 allowed for the NHMRC to retain its Council and Principal Committees (Research, Ethics); but these were now advisory to the CEO. In the case of ethics, there was retention of the provision that the Council (and now CEO) could not modify the Australian Health Ethics Committee (AHEC) guidelines, though such guidelines may be referred back to the AHEC for further consideration. Similarly, the Act preserves the NHMRC’s independence from direct detailed involvement by the Minister of the day in recommendations on the allocation of research funds or the manner in which the CEO addresses scientific, technical or ethical issues (Box).7
The revised Act also established the NHMRC as an independent agency; while no longer part of the Australian Government Department of Health and Ageing, it was still within the Health and Ageing portfolio.
The new arrangements came into force on 1 July 2006, so in a sense the NHMRC is just 6 months old. But does it have the required youthful vigour to support the best research, and to work with others to turn evidence into better health outcomes, an innovative health system and new health industries, all within the world’s best ethical frameworks? Or, as the MJA editorialist put it, will the NHMRC “become dynamic, flexible, adaptive, effective, and in tune with the 21st century”?2
Time will tell, but the NHMRC is entering this new era full of energy and ambition for what we can do to improve health in our country and internationally. Our ambition was boosted consider-ably by the government’s $905 million increase in support for health and medical research in the May 2006 budget.8 This included an additional $500 million over 4 years to the NHMRC for research into new medical knowledge and technologies with the potential to prevent and treat disease and improve the lives of Australians. The NHMRC was also awarded $170 million to create a new Australian Health and Medical Research Fellowship Scheme, to retain or attract outstanding and talented Australian researchers. The budget announcement also included $22 million towards a new national adult stem cell centre, infrastructure funding for the Walter and Eliza Hall Institute of Medical Research of $50 million, and $163 million for other health and medical research institutions. This commitment clearly indicates the government’s high expectations of Australia’s health and medical research sector.
As the newly appointed CEO, I recognise that some of the past criticisms of the NHMRC were warranted and need to be addressed. However, the NHMRC, combining research, advice and ethics into a single body, is in a stronger position than many other health research bodies internationally. Here, I discuss how the NHMRC can maximise its impact, accountability and relevance.
The world is much more complex than it was in the 1930s, when Billy Hughes said of the NHMRC “as fast as new knowledge is acquired it must be applied”.3 The gaining of knowledge is a worldwide activity, the health care sector has grown immensely in size and sophistication, and political pressures on health decision making are now much greater. Yet, this remains the area in which the NHMRC’s potential to benefit Australia remains the greatest. The Grant review contains an excellent outline of how the NHMRC can influence health policy and practice based on the best evidence.5 Commonwealth, state and territory health policymakers will be crucial partners here, through relationships with the Australian Government Department of Health and Ageing and with the states and territories, which are primarily responsible for delivering much of our health care. The NHMRC Council now has all the state and territory chief health officers as members.
This is an exciting period in health research. The NHMRC can build on Australia’s public health and health services, and its biomedical and clinical research capacities, to ensure that we have a health system able to meet emerging health challenges, as well as the increased expectations of the Australian community, our changing demography, and the greater availability of expensive but effective new treatments.
To address the question of which research to support, the NHMRC has over many years refined its peer review processes to identify the best researchers and the best projects. But not everyone agrees that the systems are optimal, with some accusing the NHMRC of using the GOBSAT method (Good Old Boys Sat Around a Table method), to quote the MJA Editor.2 Others have complained that whole areas have been excluded from support (especially new disciplines); transparency has been lacking (eg, in 2006, for a number of funding schemes, including project grants, applications were reviewed without giving applicants the opportunity to see and respond to reviewers’ comments); and, more generally, there has been a focus on process rather than outcome. To some extent, all funding bodies face similar criticisms. After all, there are never enough funds to support all worthwhile research. However, it may be relevant that none of Australia’s Nobel Prize winners in the past decade, Peter Doherty in 1996 or Robin Warren and Barry Marshall in 2005, had NHMRC support for their prize-winning research. Doherty worked at the then block-funded John Curtin School of Medical Research, but in the case of Warren and Marshall, it certainly should give us pause for thought that the NHMRC peer review process was unable to see what the Nobel Committee acknowledged subsequently. It is important therefore that the NHMRC undergoes independent scrutiny of its processes to ensure that these are fair and can identify the most meritorious research.
The NHMRC will also develop new, robust priority research processes. For this, other health research organisations, such as the US National Institutes of Health (NIH) and the Canadian Institute of Health Research, may provide models. Both have developed ways of mounting coordinated research attacks on significant issues (eg, the NIH Requests for Applications process), so that a spectrum of scientific, policy and community views can be brought to the definition of areas that require targeted research or considerable scale and scope.
A key part of the Wills review6 was the development of a vibrant industry in Australia from health and medical research. The history of many of Australia’s most successful businesses in the health sector shows the heavy dependence on the early involvement of astute business people. Think of ResMed. Colin Sullivan and his colleagues’ research was of course crucial, but it was the entrepreneurial Peter Farrell who built the successful international business. There are similar stories for Cochlear, IVF Australia, and CSL.
So what is the role of the NHMRC? A new NHMRC Management Advisory Committee will advise on improving the commercial uptake of, and strengthening industry involvement in, health and medical research. Some medical researchers may feel that social benefits from medical research are the most important. However, most Australian researchers support the thrust of the Wills review and that benefits to Australia from Australian taxpayer support of medical research should be maximised through a better standard of public health; through a better functioning health system; and through new companies, industries and services built on innovative biomedical and health research.
There are many examples of substantial philanthropic support for medical research, especially through the Victorian medical research institutes (The Kodak/Baker Foundation and the Baker Institute; the Myer family and the Howard Florey Institute). More recently, there has been generous support of two of Queensland’s research institutes (the Queensland Institute of Medical Research and the University of Queensland’s Institute of Molecular Bioscience), as well as examples in other states — the Murdoch Childrens Research Institute, the Walter and Eliza Hall Institute, and the Baker Institute, among others. Recently, universities have identified their own unique opportunities, but much more is possible.
One challenge we all face is the tendency to split the effort, with a proliferation of small institutions or foundations often competing against each other for the charitable dollar. Speaking at the National Press Club in Canberra recently, Sarah Murdoch said that the
. . . fundraising market is cluttered. Duplication of effort is rife, and the community is often confused. If we want to go forward, then action is required to rationalise and collaborate on funding for health and medical research . . .9
The NHMRC could offer potential philanthropists an important service, analogous to Moody’s or Standard and Poor’s. Our rigorous review processes each year identify the researchers and research teams that propose and conduct research that is highly worth funding.
Increasing the health and medical research expertise of the NHMRC staff was recommended by the Wills6 and Grant5 reviews and the words of this heading are again those of the MJA Editor last year.2 In the days of Billy Hughes, a part-time Council meeting twice a year may have been sufficient to fulfil the obligations of the NHMRC. However, the scope and pace of health and medical research this century requires full-time staff with clinical, health service, population health, research commercialisation, and biomedical research backgrounds. The NHMRC will shortly undertake a program of strategic recruitment of people with qualifications, skills and experience in these areas. By increasing its health and medical research professional staff, the NHMRC will be able to greatly increase its ability to respond rapidly to emerging issues, to provide insights and strategies to address pressing health issues, and to gain the most out of the Principal Committee structure of the NHMRC. The NHMRC will also introduce an intern system, whereby researchers and health care administrators can work with NHMRC staff for periods of weeks or months, to learn about the impact of the work of this diverse organisation on health in Australia. For example, interns could find themselves working on stem cell research guidelines, on an agreement with New Zealand and Canada about Indigenous health research, or on the ethics of transplant donation.
No one can predict the future, yet the NHMRC Act requires just that! It mandates a strategic plan that identifies “the major national health issues that are likely to arise” during the next 3 years.10 To ensure that Australia is able to meet the coming health challenges, we need a strong and well balanced research workforce, from biomedical, through clinical and public health research, to health services research. We also need to work at the links between researchers and research outputs and the health system, to ensure speedy and robust decision making by those administering and working in our health care system. With the ability to entwine its research, advice and ethics roles, the NHMRC is better placed than any other international health and medical research body. Now we just have to do it!
Received 24 October 2006, accepted 1 November 2006
- Warwick P Anderson1
- National Health and Medical Research Council (NHMRC), Canberra, ACT.
None identified.
- 1. Tony Abbott, Minister for Health and Ageing. New governance arrangements for NHMRC next year [press release]. 2005; 7 Sep. http://www.health.gov.au/internet/ministers/publishing.nsf/content/health-mediarel-yr2005-ta-abb108.htm/$FILE/abb108.pdf (accessed Oct 2006).
- 2. Van Der Weyden MB. Modernising the National Health and Medical Research Council [editorial]. Med J Aust 2005; 183: 340-342. <MJA full text>
- 3. Cumpston JHL. The health of the people: a study of federalism. Roebuck Society Publication No. 19. Canberra: Roebuck Society, 1978: 65-76.
- 4. Uhrig J. Review of the corporate governance of statutory authorities and office holders. Canberra: Commonwealth of Australia, 2003. http://www.finance.gov.au/governancestructures/docs/The_Uhrig_Report_July_2003.pdf (accessed Nov 2006).
- 5. Grant J (Chairman). Sustaining the virtuous cycle for a healthy, competitive Australia. Investment review of health and medical research. Final report. Canberra: Commonwealth of Australia, 2004. http://www.health.gov.au/internet/wcms/publishing.nsf/content/health-hsid-investreview (accessed Nov 2006).
- 6. Wills P (Chairman). The virtuous cycle — working together for health and medical research. Health and medical research strategic review. Canberra: Commonwealth of Australia, 1999. http://www.health.gov.au/internet/wcms/publishing.nsf/content/hmrsr.htm (accessed Nov 2006).
- 7. National Health and Medical Research Council Act 1992 (Act No. 225 of 1992 as amended), sections 5E(2)(a) and 5E(2)(b). http://www.comlaw.gov.au/ComLaw/Legislation/ActCompilation1.nsf/0/230 29FDD3FCC3FD7CA25719C008331D3/$file/NatHeaMedResCou 1992WD02.pdf (accessed Nov 2006).
- 8. Department of Health and Ageing. Funding research for future health [media release]. 2006; 9 May. http://www.health.gov.au/internet/budget/publishing.nsf/Content/budget2006-hmedia1.htm (accessed Oct 2006).
- 9. Burke N. Unite for cancer fight. Daily Telegraph (Sydney). 2006; 5 Oct.
- 10. National Health and Medical Research Council Act 1992 (Act No. 225 of 1992 as amended), section 16(1)(a). http://www.comlaw.gov.au/ComLaw/Legislation/ActCompilation1.nsf/0/23029FDD3FCC3FD7CA25719C008 331D3/$file/NatHeaMedResCou1992WD02.pdf (accessed Nov 2006).





Abstract
The National Health and Medical Research Council Act 1992 (Cwlth) was amended in 2006 to streamline governance arrangements and help the National Health and Medical Research Council (NHMRC) to become a more responsive organisation and more effective at both acquisition and implementation of new knowledge.
As part of the NHMRC’s plans for the future, we will implement the recommendations of the Investment Review of Health and Medical Research on policy- and practice-focused research, commercialisation, and recruitment of health and research professionals to the NHMRC.
The NHMRC is also improving its process for selecting and supporting the best research across biomedical, clinical, public health and health services disciplines; and will develop, trial and introduce new forms of communicating evidence-based information.