In the 2003–04 financial year, it was estimated that Australia spent 9.7% of its gross domestic product (GDP) on health, a total of $78.4 billion or about $3919 per person.1 Although there has been a shift towards deinstitutionalisation, hospitals remain the largest providers of health services in Australia, having received 34.9% of total recurrent health expenditure in 2002–03.1 Despite this, little is known about the characteristics of hospital users, particularly those who use a disproportionate share of resources (“high-cost users”).
Costs were estimated by assuming that hospitalisations had the average costs for patients in the relevant Australian Refined Diagnosis Related Group (AR-DRG), as reported in the National Hospital Cost Data Collection, WA Cost Weights Round 6 (2001–02),2 and then aggregating all costs related to the same patient. No cost data were available for seven patients, who were therefore removed from the analysis.
Total hospital separations and bed-days accrued in 2002 were aggregated for each person. For length-of-stay calculations, same-day hospital separations were counted as a length-of-stay of 1 day. Using the primary admitting diagnosis, a major diagnostic category was constructed for each person. People with multiple separations were assigned the major diagnostic category associated with the most bed-days, or the diagnostic category associated with the earliest separation when multiple diagnostic groups shared equal numbers of bed-days.3
Comorbidities were assigned using the original Charlson Index,4 which was applied to the first admission in 2002 and all hospitalisations in the previous 12 months. Coexisting diagnoses that belonged to the same ICD-10-AM chapter5 as the major diagnostic category were excluded from the comorbidity score.
High-cost users were defined as the top 5% of people when all individuals with at least one hospitalisation in 2002 were ranked from highest to lowest total inpatient cost. Defining high users on the basis of cost has been reported elsewhere.6
Age at admission was divided into nine subgroups. For each age group, two prevalence measures were calculated: (1) high-cost hospital users as a proportion of total hospital users in 2002; and (2) total hospital users as a proportion of the WA population.7 Logistic regression was used to examine the association between age (fitted as a single age-group term) and the prevalence of high-cost users in 2002. Odds ratios were calculated to examine differences in the proportion of high-cost users and other hospital users who died within 1 year of hospitalisation, and who had frequent use (≥ 12 separations) or long stay (> 35 days) hospitalisations in 2002.
Average annualised inpatient costs and bed-days were expressed as both a median and an arithmetic mean. The median provides the “typical” value for individuals, whereas the mean provides a more informative measure of the total financial impact of hospitalisation, and is more useful from a policy perspective.8
Independent samples t tests were used to compare differences in mean comorbidity score and overall resource use between high-cost users and other hospital users. Linear tests for age trend were performed for annualised resource use (cost, separations, bed-days) by entering age group as a continuous parameter in the model and adjusting for the potential confounding effects of sex, major diagnostic category, comorbidity, and living status 12 months post-hospitalisation. (This last parameter is important to avoid overemphasising the effect of age on hospital costs.)9
In large samples, t tests and least-squares linear regression are valid for any distribution, even those with very non-normal data, when the purpose of the analysis is to make inferences about the association between variables rather than to predict outcomes for individuals.10
Statistical analyses were performed using SPSS (version 12; SPSS Inc, Chicago, Ill, USA).
There were 302 788 people hospitalised (585 815 separations) in WA in 2002, excluding episodes of care that extended from 2001 or into 2003 (n = 3961 and 4276, respectively) and separations related to pregnancy (n = 40 036), newborns (n = 25 983), boarders (n = 18 043), and duplicate records (n = 6938). As three individuals had the same cost at the cut-off point (≥ $25 051), there were 15 141 high-cost users (154 433 separations), not 15 139 (Box 1).
Older people (≥ 65 years) accounted for more than half of the high-cost users (55.9%, n = 8466). The prevalence of high-cost users increased with age (odds ratio, 1.43; 95% CI, 1.42–1.44; P < 0.001). As shown in Box 1, this trend existed alongside a higher prevalence of hospital use for older people overall.
The primary diagnostic category differed between high-cost users and other hospital users (Box 2). Factors influencing health status and contact with health services was the most common reason for high-cost hospital use, of which extracorporeal dialysis and chemotherapy accounted for most separations (93.2%). Angina, heart failure and acute myocardial infarction accounted for 50.9% of separations where diseases of the circulatory system were responsible for high-cost use. Depressive illness and schizophrenia accounted for 43.5% of separations where mental and behavioural disorders were identified as the main reason for high-cost hospital use. Of the top five major diagnostic categories for high-cost users, mental and behavioural disorders was the only one in which people aged 65 years and older were not the majority. A fairly large number of inpatients (n = 361) became high-cost users as a consequence of procedural complications and complications of internal orthopaedic prosthetic devices, implants and grafts. High-cost users also had slightly higher Charlson comorbidity scores (mean difference, 0.88; 95% CI, 0.85–0.91).
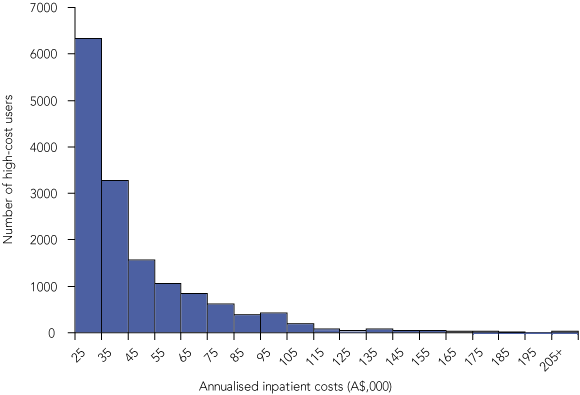
High-cost users accounted for 38.4% of both inpatient costs and inpatient days, and 26.4% of inpatient separations. They had higher annualised costs (by definition) and bed-days, which resulted in mean differences of $44 400 (95% CI, $44 274–$44 525) and 42.6 days (95% CI, 42.4–42.8 days) compared with other hospital users. The distributions for annualised costs and bed-days were positively skewed for both high-cost users and other hospital users (range of skewness coefficient, 1.8–8.4) (Box 3). High-cost users were 40 times more likely than other hospital users to be frequently hospitalised (≥ 12 separations in 2002) (95% CI, 38.5–43.5) or have a long stay hospitalisation (> 35 days) (95% CI, 37.0–41.7).
For high-cost users, costs generally decreased as age increased (mean change per age group, − $1726; 95% CI, − $1492 to − $1961; P < 0.001 for linear trend test), after adjustment for potential confounders (Box 4). This effect was most noticeable from the age of 45 years. Conversely, a significant upward trend in annualised costs was detected across age groups for other hospital users (adjusted mean change per age group, $283; 95% CI, $276–$290, P < 0.001 for linear trend test), again with the effect most noticeable from the age of 45 years. For high-cost users, the mean annualised cost for patients aged 85 years and older was $9576 (95% CI, $7687–$11 466) lower than for patients aged 45–54 years. For other hospital users, the mean annualised costs for patients aged 85 years and older was $2253 higher than for patients aged 45–54 years, after adjustment for potential confounders.
About 15% of the WA population was hospitalised in 2002 (not counting pregnancy-related events and neonates), which translated into more than half a million separations, 1.8 million hospital days, and costs of $1.9 billion. Most importantly, resource use was disproportionately concentrated in a small group of high-cost users, who comprised 5% of WA’s inpatient population, but accounted for 38% of inpatient costs and 26% of hospital days. Of obvious interest to health policy makers in search of solutions to Australia’s health care problems11 is whether this high-cost use occurs randomly and the extent to which it can be curbed.
Chronic stable and unstable conditions were a key feature of high-cost users. They also had slightly higher comorbidity scores and were nine times more likely to die in the year following their first hospitalisation (4.6 times more likely after adjustment for age). These results demonstrate vertical equity within WA’s hospital system, where people with the greatest disease burden receive the most care. This finding is consistent with Canadian research.6
Our finding that many high-cost users were hospitalised for chronic conditions (such as end-stage renal disease, angina, congestive heart failure and cancer) that have identifiable and modifiable risk factors highlights the potential preventability of some hospital use. Key risk factors for chronic renal failure, the most common cancers, and circulatory disease overlap considerably, and include type 2 diabetes, obesity, hypertension, tobacco use, alcohol consumption, dyslipidaemia, sedentary lifestyle, and poor diet.12-14 Effective strategies for addressing these risk factors have been reported, highlighting the considerable opportunity for reducing medical costs and human suffering through prevention strategies that focus on lifestyle modification and pharmacological management.14-17 However, widespread behaviour change must be facilitated by intervention at multiple levels, requiring regulatory and environmental change.
In other areas, evidence supporting the effectiveness of preventive strategies is lacking or mixed. For example, there have been many falls prevention initiatives to reduce fractured femurs in older people (eg, home hazards modifications, exercise programs), but limited evidence of their effectiveness.18,19
People with mental and behavioural disorders comprised 14.1% of high-cost users. Furthermore, of the top five primary conditions of high-cost users, this was the only one not dominated by older people. Worldwide, policies have supported the development of community care initiatives intended to avoid hospitalisation of people with severe mental disorders. Cochrane reviews of the effectiveness of these interventions in reducing hospital use and health care costs are abundant, and with few exceptions, the results are far from promising,20-23 if not altogether lacking.24 In a more recent WA study, Preston and colleagues concluded that compulsory community-based psychiatric treatment does not reduce the use of health services.25 The reality is that, for some people with severe mental disorders, hospital may provide the most appropriate treatment available and efforts to curb use may prove both futile and counterproductive to achieving good health outcomes (eg, treatment compliance, suicide reduction, reduced caregiver burden).
We found that 361 patients were classified as high-cost users because of complications of internal orthopaedic prosthetic devices, implants and grafts, or complications not elsewhere classifiable. Perhaps high-cost users had underlying risk factors that predisposed them to complications, for example, older age, severity of illness or the complexity of care required.26 Apart from patient factors, there is also a need to assess how many of these complications resulted from medical errors, as a result of substandard performance, products, procedures or organisational systems.27 Developing confidential, objective, complete and non-punitive reporting systems and review processes is essential if we are to identify preventable complications, remedy their root causes, and reduce medical costs.28
This study was limited in several aspects. First, we excluded people with hospital stays exceeding 1 year. Second, there may be residual confounding by comorbidity, because the Charlson Index was not designed for a paediatric population. Lastly, the use of administrative records may have underestimated the burden of disease.29
2 Top five diagnostic categories responsible for the most hospital days per year, Western Australia, 2002
Received 26 October 2005, accepted 14 February 2006
- Janine Calver1,2
- Kate J Brameld3
- David B Preen3
- Stoney J Alexia3
- Duncan P Boldy1
- Kieran A McCaul4
- 1 Centre for Research into Aged Care Services, Curtin University of Technology, Perth, WA.
- 2 Information Collection and Management, WA Department of Health, Perth, WA.
- 3 School of Population Health, University of Western Australia, Perth, WA.
- 4 School of Public Health, Curtin University of Technology, Perth, WA.
This project was made possible by the technical expertise of the Western Australian Data Linkage Unit in the WA Department of Health. We thank the WA Department of Health for funding this study.
None identified.
- 1. Australian Institute of Health and Welfare. Health expenditure Australia 2003–04. Health Expenditure Series No. 25. Canberra: AIHW, 2005. (AIHW Catalogue No. HWE 32.) Available at: http://www.aihw.gov.au/publications/index.cfm/title/10204 (accessed Mar 2006).
- 2. Australian Government Department of Health and Ageing. National hospital cost data collection. WA cost weights round 6 (2001–02). Available at: http://www.health.gov.au/internet/wcms/Publishing.nsf/Content/health-casemix-costing-fc_r6.htm (accessed Apr 2005).
- 3. Roos N, Burchill C, Carriere K. Who are the high hospital users? A Canadian case study. J Health Serv Res Policy 2003; 8: 5-10.
- 4. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40: 373-383.
- 5. National Centre for Classification in Health. The international statistical classification of diseases and related health problems, 10th revision, Australian modification (ICD-10-AM). Sydney: National Centre for Classification in Health, 1998.
- 6. Reid R, Evans R, Barer M, et al. Conspicuous consumption: characterizing high users of physician services in one Canadian province. J Health Serv Res Policy 2003; 8: 215-224.
- 7. Australian Bureau of Statistics. Population estimates by age and sex, Western Australia, 2002. Canberra: ABS, 2002. (ABS Catalogue No. 3235.0.55.001.)
- 8. Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000; 320: 1197-1200.
- 9. Gray A. Population ageing and health care expenditure. Ageing Horizons 2005; (2): 15-20.
- 10. Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health 2002; 23: 151-169.
- 11. Van Der Weyden MB. Australian healthcare: purposeful reform or three more years of political rhetoric? [editorial] Med J Aust 2004; 181: 593-594. <MJA full text>
- 12. Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases. Part I: general considerations, the epidemiologic transition, risk factors and the impact of urbanization. Circulation 2001; 104: 2746-2753.
- 13. Stein C, Colditz G. Modifiable risk factors for cancer. Br J Cancer 2004; 90: 299-303.
- 14. Nahus A, Bello A. Chronic kidney disease: the global challenge. Lancet 2005; 365: 331-340.
- 15. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998; 352: 837-853. Correction in Lancet 1999; 354: 602.
- 16. Gluckman T, Baranowski B, Ashen M, et al. A practical and evidence-based approach to cardiovascular disease risk reduction. Arch Intern Med 2004; 164: 1490-1500.
- 17. Goldstein MG, Whitlock EP, DePue J, et al. Multiple behavioural risk factor interventions in primary care: summary of research evidence. Am J Prev Med 2004; 27(2S): 61-79.
- 18. Gillespie LD, Gillespie WJ, Roberston MC, et al. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev 2003; (4): CD000340.
- 19. Lyons RA, Sander LV, Weightman AL, et al. Modification of the home environment for the reduction of injuries. Cochrane Database Syst Rev 2003; (4): CD003600.
- 20. Marshall M, Gray A, Lockwood A, et al. Case management for people with severe mental disorders. Cochrane Database Syst Rev 2000; (2): CD000050.
- 21. Tyrer P, Coid J, Simmonds S, et al. Community mental health teams (CMHTs) for people with severe mental illness and disordered personality. Cochrane Database Syst Rev 2000; (4): CD000270.
- 22. Joy CB, Adams CE, Rice K. Crisis intervention for people with severe mental illnesses. Cochrane Database Syst Rev 2004; (4): CD001087.
- 23. Marshall M, Crowther R, Almaraz-Serrano A, et al. Day hospital versus admission for acute psychiatric disorders. Cochrane Database Syst Rev 2003; (1): CD004026.
- 24. Catty J, Burns T, Comas A. Day centres for severe mental illness. Cochrane Database Syst Rev 2001; (2): CD001710.
- 25. Preston NJ, Kisely S, Xiao J. Assessing the outcome of compulsory psychiatric treatment in the community: epidemiological study in Western Australia. BMJ 2002; 324: 1244.
- 26. Thomas EJ, Brennan TA. Incidence and types of preventable adverse events in elderly patients: population based review of medical records. BMJ 2000; 320: 741-744.
- 27. Edmonds M. Adverse events, iatrogenic injury and error in medicine. Adelaide: University of Adelaide, 2005. Available at: http://www.informatics.adelaide.edu.au/topics/Safety/ME-AdverseEvents.html (accessed Apr 2005).
- 28. Barach P, Small S. Reporting and preventing medical mishaps: lessons from non-medical near miss reporting systems. BMJ 2000; 320: 759-763.
- 29. Preen DB, Holman CDJ, Lawrence DM, et al. Hospital chart review provided more accurate comorbidity information than data from a general practitioner survey or an administrative database. J Clin Epidemiol 2004; 57: 1295-1304.





Abstract
Objective: To describe how high-cost users of inpatient care in Western Australia differ from other users in age, health problems and resource use.
Design and data sources: Secondary analysis of hospital data and linked mortality data from the WA Data Linkage System for 2002, with cost data from the National Hospital Cost Data Collection (2001–02 financial year).
Outcome measures: Comparison of high-cost users and other users of inpatient care in terms of age, health profile (major diagnostic category) and resource use (annualised costs, separations and bed days).
Results: Older high-cost users (≥ 65 years) were not more expensive to treat than younger high-cost users (at the patient level), but were costlier as a group overall because of their disproportionate representation (n = 8466; 55.9%). Chronic stable and unstable conditions were a key feature of high-cost users, and included end stage renal disease, angina, depression and secondary malignant neoplasms. High-cost users accounted for 38% of both inpatient costs and inpatient days, and 26% of inpatient separations.
Conclusion: Ageing of the population is associated with an increase in the proportion of high-cost users of inpatient care. High costs appear to be needs-driven. Constraining high-cost inpatient use requires more focus on preventing the onset and progression of chronic disease, and reducing surgical complications and injuries in vulnerable groups.