Although clinical trials of the antipsychotic amisulpride revealed no cardiac adverse effects, four patients with severe cardiac toxicity after overdose were reported to Australian poisons information centres in 2004–2005. All four had QT prolongation over 500 ms, two had rate-dependent bundle branch block, two developed torsades de pointes, and one died after cardiac arrest. Pending further studies, we recommend electrocardiogram assessment until at least 16 h after amisulpride overdose and, if QT interval is prolonged, cardiac monitoring until the patient is clinically well and conduction intervals are normal.
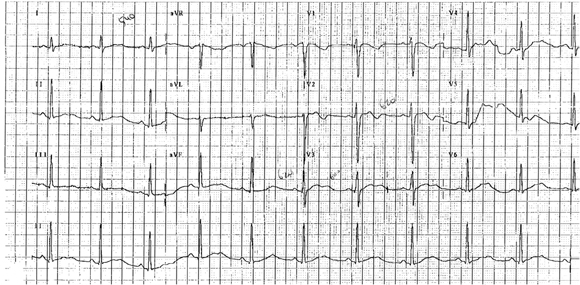
On arrival, 7 h after the overdose, her condition remained unchanged. An electrocardiogram (ECG) showed sinus rhythm, heart rate of 67 bpm, QRS interval of 128 ms, prolonged QT interval of 560 ms and bifid T waves (Box 1). Twelve hours after ingestion, her level of consciousness decreased (GCS, 4), and broad complex tachycardia was observed on the electrocardiography monitor and subsequent ECG. No hypotension was recorded. She was intubated, hyperventilated, given NaHCO3, magnesium and calcium gluconate, and transferred to the intensive care unit. The QRS interval narrowed to 112 ms within 4 h, but the QT interval remained prolonged for another 12 h.
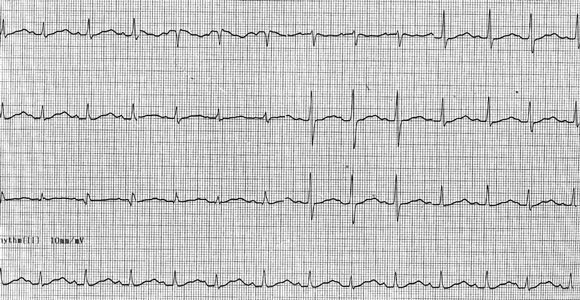
A 40-year-old man presented to a hospital emergency department after ingesting amisulpride (32 g), mirtazapine (300mg), valproate (7 g), amitriptyline (1.25 g) and omeprazole (unknown quantity). On arrival, he had a GCS of 14, heart rate of 90 bpm, and blood pressure of 120/70mmHg. An ECG at presentation showed sinus rhythm with a heart rate of 90 bpm, QT interval of 460ms and bifid T waves. He was admitted to the intensive care unit. About 12.5 h after ingestion, he developed a broad complex tachycardia with rate 120 bpm (left bundle branch pattern), but remained haemodynamically stable. The QRS complex did not significantly narrow when the patient was treated with a bolus of NaHCO3. An NaHCO3 infusion was started, and he was intubated and ventilated. Eighteen hours after ingestion, the QT interval was 560ms, with heart rate of 79 bpm and a normal QRS interval (Box 2A).
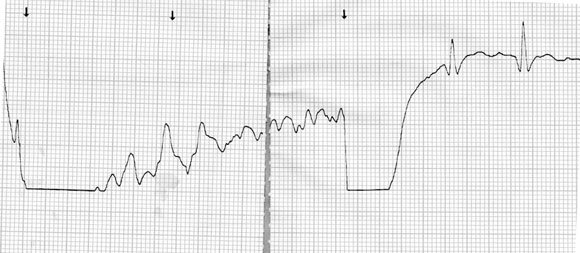
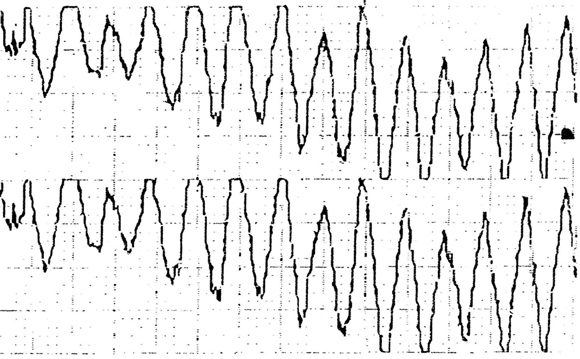
About 29 h after ingestion, the patient developed pulseless torsades de pointes, but sinus rhythm with a QT interval of 560ms was restored after a single direct current cardioversion shock (Box 2B). He had a second episode of torsades de pointes 32.5 h after ingestion, and an episode of ventricular tachycardia 34 h after ingestion. By 5 days after the overdose, the QT interval had shortened to 360ms (Box 2C).
Serum amisulpride level was measured by high performance liquid chromatography using a modified method of Bohbot et al,1 and was 23.2 mg/L at 12.5 h after ingestion.
A 22-year-old man presented to hospital 2 h 20 min after ingesting amisulpride (4.6 g). There was no family history of sudden death or cardiac arrhythmias. On arrival, he was alert and oriented, had a heart rate of 69 bpm, and blood pressure of 136/61 mmHg. The ECG showed sinus rhythm, heart rate of 63 bpm, and QT interval of 600 ms (Box 3A). Repeat ECGs showed QT intervals between 580 ms and 640 ms.
Seven hours after ingestion, he had an episode of pulseless torsades de pointes (Box 3B). Cardioversion was achieved with a 200 J direct current shock. As the QT interval remained prolonged (600 ms), therapy with isoprenaline (60 μg/h) was begun. After an hour, the dose was decreased to 30 μg/h and continued for 20 h.
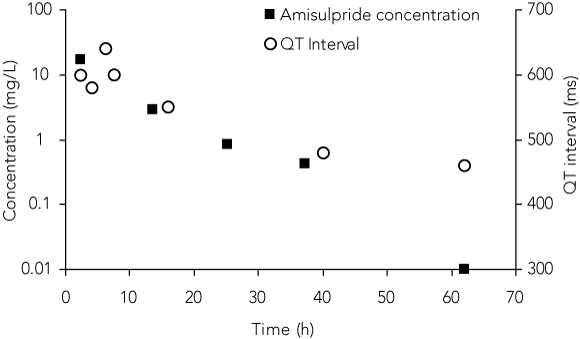
The patient had a second episode of torsades de pointes almost 24 h after ingestion. This was asymptomatic and resolved spontaneously within 30 seconds. He had bradycardia (heart rate, 40 bpm) for 24 h after isoprenaline was ceased. The QT interval gradually decreased to 460 ms at 62 hours after ingestion and was 360 ms 3 weeks later (Box 3C). Box 3D illustrates the time course of serum amisulpride concentration and absolute QT interval over the first 60 h after ingestion.
Amisulpride is an antipsychotic that has been available on the Pharmaceutical Benefits Scheme in Australia since 2003. A benzamide derivative, it is well tolerated, with relatively few side effects and minimal behavioural toxicity in doses with antipsychotic effect.2 Amisulpride overdoses were first reported to Australian poisons information centres in early 2004, and about 60 telephone calls about amisulpride overdose were made from hospitals to the New South Wales and Western Australian Poisons Information Centres between July 2004 and June 2005, including the four cases reported here.
A review of clinical trials of amisulpride reported that it had no effect on the ECG in therapeutic doses and did not produce arrhythmias.3 There are no published data on animal toxicity. It was therefore surprising that such severe effects were seen in overdose. There were a few published reports of overdose in the literature before its introduction in Australia,4,5 and a reference to QT prolongation and torsades de pointes in the manufacturers product information, but little indication that overdose could have effects as severe as those reported here, including the first cases of torsades de pointes.
During the past decade, there have been sporadic reports of amisulpride poisoning, including two deaths.4-9 Significant QT prolongation was reported in a recent series of eight cases with limited clinical details9 and in another two cases, where it was suggested to be related to hypocalcaemia.7 A patient who suffered multiple cardiac arrests has also been reported. Although torsades de pointes was suspected, it was not confirmed on ECG.6
These cases demonstrate that amisulpride overdose may be associated with clinically significant QT prolongation. The absolute QT interval was over 500ms in all four of our cases and close to 600ms in three. Amisulpride overdose was associated with ECG-confirmed torsades de pointes in two cases and a cardiac arrest resulting in death in another. Unfortunately, no ECG was available to determine if torsades de pointes had occurred in that patient. The magnitude of the effect on the QT interval and the number of cases of torsades de pointes from about 60 cases of overdose reported to the Poisons Information Centres suggests that amisulpride overdose is associated with significant cardiac toxicity. Amisulpride also caused a ratedependent bundle branch block in two cases. Although this coincided with a decreased level of consciousness, it was unlikely to have caused it. Drowsiness occurred in three of the four patients and profound sedation in two, suggesting that amisulpride also causes central nervous system depression.
Amisulpride was detected in high concentrations in three of our patients — three to four orders of magnitude above that reported in therapeutic studies.2 In these studies, the peak concentration after a dose of 50mg of amisulpride was 55.7 µg/L (SD, 3.7).
Citalopram is another drug that appeared to cause minor or no cardiac effects in clinical trials of therapeutic doses,10 but in overdose has been associated with moderate QT prolongation,11,12 rate-dependent bundle branch block and, rarely, torsades de pointes.13,14 A pharmacokinetic and pharmacodynamic model of citalopram intoxication clearly demonstrated a dose-dependent relationship between drug concentration and QT interval.12 Fortunately, citalopram-associated torsades de pointes appears very rare, with only two published reports.
2 Electrocardiogram changes in Patient 2
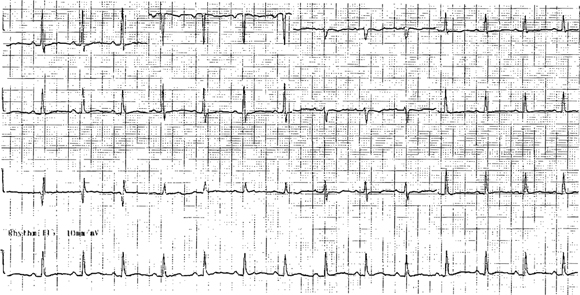
C: Five days after the overdose, ECG showed shortening of QT to 360 ms. |
- 1. Bohbot M, Doare L, Diquet B. Determination of a new benzamide, amisulpride, in human plasma by reversed-phase ion-pair high-performance liquid chromatography. J Chromatogr 1987; 416: 414-419.
- 2. Rosenzweig P, Canal M, Patat A, et al. A review of the pharmacokinetics, tolerability and pharmacodynamics of amisulpride in healthy volunteers. Hum Psychopharmacol 2002; 17: 1-13.
- 3. Coulouvrat C, Dondey-Nouvel L. Safety of amisulpride (Solian): a review of 11 clinical studies. Int Clin Psychopharmacol 1999; 14: 209-218.
- 4. Dorne R, Pommier C, Manchon M, Berny C. Intoxication with amisulpride (Solian): a case with toxicologic documentations. Therapie 2000; 55: 325-328.
- 5. Tracqui A, Mutter-Schmidt C, Kintz P, et al. Amisulpride poisoning: a report on two cases. Hum Exp Toxicol 1995; 14: 294-298.
- 6. Eleouet C, Lichtenstein D, Delhotal-Landes B, Flouvat B. Contribution of emergency toxicology in a case of amisulpride poisoning [abstract]. Eur J Emerg Med 2001; 8: 70.
- 7. Ward DI. Two cases of amisulpride overdose: a cause for prolonged QT syndrome. Emerg Med Australas 2005; 17: 274-276.
- 8. Musshoff F, Kroner L, Padosch SA, Madea B. Fatal intoxication with amisulpride and presentation of organ distribution. Arch Kriminol 2005; 215: 158-163.
- 9. Pehourcq F, Ouariki S, Begaud B. Rapid high-performance liquid chromatographic measurement of amisulpride in human plasma: application to manage acute intoxication. J Chromatogr B Analyt Technol Biomed Life Sci 2003; 789: 101-105.
- 10. Rasmussen SL, Overo KF, Tanghoj P. Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol 1999; 19: 407-415.
- 11. Isbister GK, Bowe SJ, Dawson A, Whyte IM. Relative toxicity of selective serotonin reuptake inhibitors (SSRIs) in overdose. J Toxicol Clin Toxicol 2004; 42: 277-285.
- 12. Friberg LE, Isbister GK, Duffull SB. Pharmacokinetic pharmacodynamic modelling of QT interval prolongation following citalopram overdoses. Br J Clin Pharmacol 2006; 61: 177-190.
- 13. Tarabar AF, Hoffman RS, Nelson LS. Citalopram overdose: late presentation of torsades de pointes (TdP) with cardiac arrest [abstract]. J Toxicol Clin Toxicol 2003; 41: 676.
- 14. Meuleman C, Jourdain P, Bellorini M, et al. Citalopram and torsades de pointes. A case report. Arch Mal Coeur Vaiss 2001; 94: 1021-1024.






None identified.