In Victoria in 2003, there were 346 cases of primary brain and central nervous system cancers with 341 deaths, making it the eighth most common cause of cancer-related death in Victoria.1 The majority of these cancers are high-grade gliomas: glioblastoma multiforme (GBM), anaplastic astrocytoma and mixed anaplastic astrocytoma/oligodendroglioma. For patients with glioma, poorer prognosis is associated with increasing tumour grade, older age, worse performance status and residual disease after surgical resection.2-4
The standard approach to treatment of gliomas depends on the tumour grade. There are published US and European management guidelines for gliomas, but there are no Australian guidelines.5,6 Further, little is known about current treatment practices in Australia. A recent study of glioma management across 52 US institutions demonstrated wide variation in the therapeutic approach to high-grade gliomas.7
Over the period 1998–2000, 992 glioma cases were identified from the VCR, of which 890 were eligible for our study. Completed questionnaires were obtained for 828 (93%) of the eligible cases (Box 1), with 695 (84%) of questionnaires completed by the survey data manager from clinicians’ medical histories.
Patient characteristics are summarised in Box 2. There was a slight male preponderance. Just over half the patients were aged over 60 years, 264 (32%) over 70 years and 92 (11%) over 80 years. The median age of all patients was 72 years (range, 16–85 years). Tumour grade was determined in 654 patients (86%) who underwent biopsy or resection. A further 69 patients (8%) had a diagnosis of glioma without a grade specified. A significant proportion of ungraded tumours were the result of inadequate samples being taken from critical areas (including spinal cord and brainstem). Seventy-one per cent of tumours that were graded were GBM. Not all reports were from specialist neuropathologists.
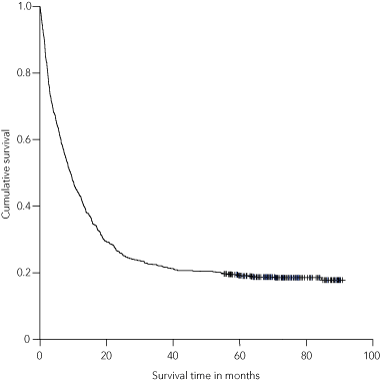
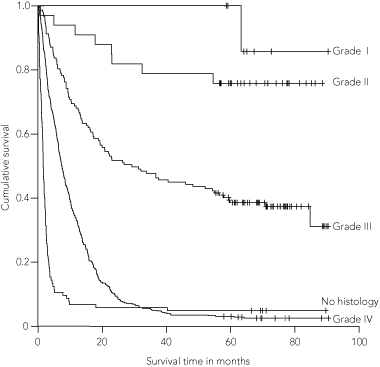
The overall survival times for all patients and for patients according to tumour grade are shown in Box 4. The median survival was 9.2 months (range, 0–84+ months) overall and 7.4 months (range, 0–84+ months) in patients with GBM. The 5-year survival rate was 19% for the entire cohort and 3% for patients with GBM.
Our study documents many expected features in this patient group. At least half the patients were over 60 years of age and the majority had high-grade gliomas. As the Australian population ages, it is likely that more elderly patients with gliomas will be diagnosed. However, most management strategies have been based on younger patients and may not be appropriate for older patients because of more severe side-effects in older people.5,6
The general approach to management conformed to recent standards of care. That is, histological diagnosis was obtained, a macroscopic resection was performed (if feasible), patients were referred for radiotherapy and then received chemotherapy, either in an adjuvant fashion or at disease recurrence.4,8-10
However, several observations were of concern. Firstly, 13% of patients did not have a histological diagnosis and 23% had only a biopsy performed. The reasons for this were explored and generally reflected the patient’s age, comorbidities, location of the lesion and patient refusal. Secondly, only 74% of patients were referred after initial diagnosis to a radiation oncologist and only 54% were referred to a neuro-oncologist. Thirdly, 12% of patients had no active anticancer therapy such as surgery, radiotherapy or chemotherapy. Fourthly, it appeared that few patients were referred for rehabilitation, allied health evaluation/support or psychiatric/psychological assessment. Finally, only 5% of patients were enrolled onto a clinical trial. This is similar to the overall rate of about 6% for all cancer types in the state of Victoria (Susan Fitzpatrick, Executive Officer, Centre for Clinical Research in Cancer, Cancer Council of Victoria, personal communication) but lower than the 15% rate observed in the US Glioma Outcomes Project.11
Chang and colleagues recently reported a “patterns of care” survey similar to ours for 565 patients with malignant gliomas7 — to date the largest such survey reported. A comparison of their findings with ours (Box 5) demonstrates very similar outcomes in the group of patients with GBM, apart from a lower rate of radiotherapy in our patients.
Our study has several limitations. Firstly, the very nature of the study design, a retrospective cohort study, will result in some biases and inaccuracies. Secondly, not all questionnaires were returned and we cannot speculate on the data relating to unreported patients. Thirdly, a proportion of patients who never had a biopsy confirmation of glioma may have had a non-glioma disease. Fourthly, the accuracy of the extent of resection estimated by the surgeon is unreliable.12 Fifthly, there may be considerable under-reporting of the number of patients referred to allied health professionals. Finally, the cohort represents a period of 3 years, during which management practice may have changed. Indeed, it is now over 8 years since some of these patients were diagnosed and treated. However, we believe this is an important body of information regarding glioma management in Australia. It also establishes the “standard of care” during this period and will allow us to observe changes in practice.
The management of gliomas has become even more complex since the completion of our study, with a number of significant changes in treatment practice. The role of palliative chemotherapy (temozolomide) for recurrent high-grade gliomas has been established.13-15 Concurrent radiotherapy and temozolomide therapy has now become the standard treatment for patients with newly diagnosed GBM.16 In addition, the technology available to both surgeons and radiation oncologists has improved significantly. Furthermore, clinicians now have access to improved imaging techniques such as early postoperative magnetic resonance imaging and magnetic resonance spectroscopy, thallium scanning and positron emission tomography.
1 Number of completed responses to primary questionnaire and reasons for non-inclusion in our study*
* Based on 992 cases identified from the Victorian Cancer Registry. |
|||||||||||||||
Received 29 November 2005, accepted 5 January 2006
- Mark A Rosenthal1
- Katharine J Drummond2
- Michael Dally3
- Michael Murphy4
- Lawrence Cher5
- David Ashley6
- Vicky Thursfield7
- Graham G Giles8
- 1 Royal Melbourne Hospital, Melbourne, VIC.
- 2 William Buckland Radiotherapy Centre, Alfred Hospital, Melbourne, VIC.
- 3 St Vincent’s Hospital, Melbourne, VIC.
- 4 Austin Health, Melbourne, VIC.
- 5 Royal Children’s Hospital, Melbourne, VIC.
- 6 Cancer Epidemiology Centre, Cancer Council of Victoria, Melbourne, VIC.
We gratefully acknowledge the support of the Cancer Council of Victoria, the Neuro-Oncology Committee of the Victorian Cooperative Oncology Group, the Victorian Cancer Registry, and the clinicians who completed the survey.
None identified.
- 1. Thursfield V, Farrugia H, Billiet D, Giles G, editors. Cancer in Victoria 2003. Melbourne: The Cancer Council Victoria, 2004. (Canstat Series No. 41.)
- 2. Burton EC, Prados MD. Malignant gliomas. Curr Treat Options Oncol 2000; 1: 459-468.
- 3. Lacroix M, Abi-Said D, Fourney D, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg 2001; 95: 190-198.
- 4. Sawaya R. Extent of resection in malignant glioma: a critical summary. J Neurooncol 1999; 42: 303-305.
- 5. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. 2005. Available at: http://www.nccn.org/professionals/physician_gls/default.asp (accessed Feb 2006).
- 6. Stupp R, Pavlidis N, Jelic S; European Society of Medical Oncology Guidelines Task Force. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol 2005; 16 Supp l: i64-i65.
- 7. Chang SM, Parney IF, Huang W, et al. Patterns of care for adults with newly diagnosed malignant glioma. JAMA 2005; 293: 557-564.
- 8. Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet 2002; 359: 1011-1018.
- 9. Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Gliomas Outcome Project. Neurosurgery 2004; 54: 358-366.
- 10. Laperriere N, Zuraw L, Cairncross G; Cancer Care Ontario Practice Guidelines Initiative Neuro-Oncology Disease Site Group. Radiotherapy for newly diagnosed malignant glioma in adults: a systematic review. Radiother Oncol 2002; 64: 259-273.
- 11. Chang SM, Barker FG, Schmidt MH, et al. Clinical trial participation among patients enrolled in the Glioma Outcomes Project. Cancer 2002; 94: 2681-2687.
- 12. Albert FK, Forsting M, Sartor K, et al. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on re-growth and prognosis. Neurosurgery 1994; 34; 45-61.
- 13. Harris MT, Rosenthal MA, Ashley DL, Cher L. An Australian experience with temozolomide for the treatment of recurrent high-grade gliomas. J Clin Neurosci 2001; 8: 325-327.
- 14. Yung KA, Albright R, Olson J, et al. A phase II study of temozolomide vs procarbazine in glioblastoma multiforme at first relapse. Br J Cancer 2000; 83: 588-593.
- 15. MacDonald DR, Kiebert G, Prados M, Yung A, Olson J. Benefit of temozolomide compared with procarbazine in treatment of glioblastoma multiforme at first relapse: effect on neurological functioning, performance status, and health related quality of life. Cancer Invest 2005; 23: 138-144.
- 16. Stupp R, Mason WP, van den Bent ML, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352: 987-996.





Abstract
Objective: To describe the management of and outcomes in a population-based cohort of patients with newly diagnosed glioma.
Design, setting and patients: Retrospective cohort study of patients with glioma newly diagnosed over the period 1998–2000 in Victoria. Patients were identified from the population-based Victorian Cancer Registry (VCR). Doctors involved in managing the patients were surveyed by a questionnaire sent out in 2003. The cohort was followed until the end of 2004 to obtain at least 4 years’ follow-up data on all patients.
Main outcome measures: Reported treatment, referral patterns and survival rates.
Results: Over the study period, 992 cases of glioma were identified; 828 completed surveys on eligible patients were obtained (response rate, 93%); 473 patients (57%) had glioblastoma multiforme (GBM); 105 patients (13%) diagnosed with “glioma” had had no histological confirmation. Complete macroscopic resection was performed in 209 patients (25%); 612 patients (74%) were referred for radiotherapy and 326 (54%) for chemotherapy; 39 (5%) were enrolled on a clinical trial. Median survival was 9.2 months for all patients and 7.4 months for patients with GBM.
Conclusions: This is the largest reported glioma management survey in the world to date. Much of the patient demographics and approach to treatment were as expected and represent a reasonable “standard of care”. However, there are some areas for improvement, including the absence of histological diagnosis in some patients, lack of multidisciplinary care, low clinical trial enrolment and poor use of ancillary services.