Microscopic colitis is increasingly recognised as a major cause of persistent diarrhoea.1 It is an idiopathic clinicopathological syndrome of chronic watery non-bloody diarrhoea associated with a normal appearance on colonoscopy and specific histopathological changes of lymphocytic and/or collagenous colitis. There is a high rate of spontaneous resolution and relapse in microscopic colitis, and effective treatment is limited.
The pathogenesis of microscopic colitis is poorly understood and is thought to be related to a poorly regulated epithelial immune response to luminal or epithelial antigens including bile acids, toxins, or infectious agents.2 Microscopic colitis has been associated with autoimmune diseases and with exposure to medications, predominantly non-steroidal anti-inflammatory drugs, and, rarely, salicylates, simvastatin, ticlopidine, ranitidine, carbamazepine, Cyclo 3 Fort (a combination of Ruscus aculeatus extract, hesperidin methylchalcone, and ascorbic acid), flutamide, gold salts, and, recently, lansoprazole.2
Lymphocytic and collagenous colitis are probably aetiologically related, and may be a spectrum of the same disease.3 This is supported by our report of three lansoprazole-associated cases, in which one showed lymphocytic colitis, one showed collagenous colitis and one showed transitional features of both on histopathological examination. The inflammatory infiltrate and collagen deposition in these cases was less severe than that seen in typical cases of lymphocytic and collagenous colitis, probably because of the limited periods of exposure to the inciting agent. Interestingly, Patient 2 had the greatest collagen deposition and the longest exposure to lansoprazole. The rapid resolution of the symptoms on cessation of lanzoprazole therapy in Patient 1 may be related to the very mild lymphocytic infiltrate and lack of collagen deposition, or perhaps lansoprazole caused diarrhoea through another mechanism in this case.
Adverse drug reactions can only be recognised if doctors maintain a high index of suspicion. It is not possible to know every possible reaction to the medications that patients are exposed to, and some may not have been recognised before. The absence of an immediate temporal relationship between a drug and the associated disorder (as was seen in the cases presented here) may contribute to failure to diagnose a drug-induced disease.
Drugs and their metabolites may affect the colon directly through their pharmacological actions, or through hypersensitivity reactions. Drugs also act indirectly on the colon by altering colonisation by gastrointestinal organisms.4 The association between lansoprazole and microscopic colitis may be related to its action on the colonic proton pump, affecting colonic secretions and pH, which may affect colonic flora and bile salt solubility.5 Alternatively, there may be an idiosyncratic direct hypersensitivity reaction by the colonic mucosa.
The association between lansoprazole and microscopic colitis has been reported previously,5-7 and before our patients presented, the Adverse Drug Reaction Advisory Committee had received a single report of a probable association between rabeprazole and unspecified colitis. In a previous report of lansoprazole-associated microscopic colitis,5 substituting omeprazole for lansoprazole did not lead to recurrent diarrhoea. One explanation for the absence of a class effect is that while all proton-pump inhibitors bind the parietal cell proton pump covalently at cysteine 813 or 822, only lansoprazole and rabeprazole bind to cysteine 321.8 Binding of cysteine 321 also inhibits the colonic proton pumps.9 This may affect colonic secretion and pH, predisposing to diarrhoea and microscopic colitis. However, the rarity of the association between lansoprazole and microscopic colitis favours an idiosyncratic immune reaction. Minor variations in the structures of the proton-pump inhibitors may result in different immunological activation, although allergic cross-sensitivity has occasionally been reported among the proton-pump inhibitors.10
Our cases illustrate the importance of considering exposure to medication as a cause of chronic diarrhoea and particularly microscopic colitis, and that adverse drug reactions are not always class effects. Stopping the causative medication may reverse the abnormality in this difficult-to-treat condition. In patients with persistent watery diarrhoea, biopsies should be performed even if colonoscopic appearances are normal, and pathologists should be informed of the clinical possibility of microscopic colitis, as the histological changes may be subtle.
- 1. Nielsen OH, Vainer B, Schaffalitzky de Muckadell OB. Microscopic colitis: a missed diagnosis? Lancet 2004; 364: 2055-2057.
- 2. Tagkalidis P, Bhathal P, Gibson P. Microscopic colitis. J Gastroenterol Hepatol 2002; 17: 236-248.
- 3. Baert F, Wouters K, D’Haens G, et al. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut 1999; 45: 375-381.
- 4. Price AB. Pathology of drug-associated gastrointestinal disease. Br J Clin Pharmacol 2003; 56: 477-482.
- 5. Mukherjee S. Diarrhea associated with lansoprazole. J Gastroenterol Hepatol 2003; 18: 602-603.
- 6. Thomson RD, Lestina LS, Bensen SP, et al. Lansoprazole-associated microscopic colitis: a case series. Am J Gastroenterol 2002; 97: 2908-2913.
- 7. Wilcox GM, Mattia A. Collagenous colitis associated with lansoprazole. J Clin Gastroenterol 2002; 34: 164-166.
- 8. Besancon M, Simon A, Sachs G, Shin JM. Sites of reaction of the gastric H,K-ATPase with extracytoplasmic thiol reagents. J Biol Chem 1997; 272: 22438-22446.
- 9. Kaunitz JD, Sachs G. Identification of a vanadate-sensitive potassium-dependent proton pump from rabbit colon. J Biol Chem 1986; 261: 14005-14010.
- 10. Natsch S, Vinks MH, Voogt AK, et al. Anaphylactic reactions to proton-pump inhibitors. Ann Pharmacother 2000; 34: 474-476.





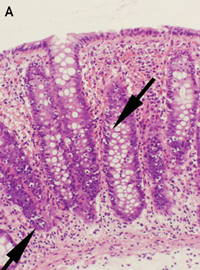
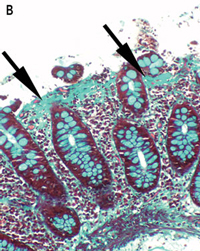
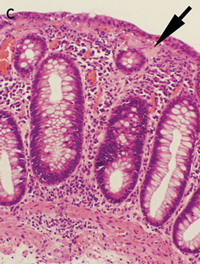
None identified.