Androgen deprivation therapy (ADT), with gonadotropin-releasing hormone analogues or surgical orchidectomy, is standard treatment for advanced prostate cancer. This intervention results in significant improvements in survival when used early in men with locally advanced prostate cancer,1,2 in men with locally advanced prostate cancer treated with radiation therapy,3 and in men with lymph-node-positive prostate cancer treated with radical prostatectomy and pelvic lymphadenectomy.4 ADT is also used in patients whose cancer relapses after curative therapy with either prostatectomy or radiation therapy, although there is no available data on survival benefit. In Australia, we have estimated from Health Insurance Commission data for the financial year 2003–04 that over 16 000 men are receiving ADT with gonadotropin-releasing-hormone antagonists.5 This number was determined by dividing the prescription number by the appropriate interval (1 month, 3 months, 4 months). All of the 3-monthly and 4-monthly prescriptions were assumed to be men with prostate cancer, and of the 1-monthly prescriptions, we assumed that half were for men.
Prostate cancer remains one of the most common causes of cancer-related death in men. However, as patients survive for lengthy periods after diagnosis, interest is now focused on the long-term adverse effects of ADT — loss of bone mineral density (BMD) and increase in fracture risk.
We present an update on assessing bone health in patients with prostate cancer who are being treated with ADT. Research papers were identified from MEDLINE and CINAHL, with combinations of the keywords androgen deprivation therapy, gonadotropin-releasing-hormone analogues, prostate cancer, osteoporosis and bisphosphonates. We also used the Cochrane database. Our purpose was to evaluate the impact of ADT on osteoporosis and fracture rates in the absence of known metastatic bone disease. Our findings are graded according to National Health and Medical Research Council levels of evidence.6 We conclude with recommendations for monitoring and treating osteoporosis in patients with prostate cancer who are to commence ADT.
Osteoporosis with fracture is associated with significant morbidity and loss of independence. Mortality after hip fracture is as high as 32% at 12 months in patients aged over 70 years, and men are much less likely to be started on bisphosphonate treatment than women.7 The incidence of osteoporosis in Australian men, although much lower than in women, is not negligible; it ranges from 1.6% in men aged 60–64 years up to 19% in those aged over 80 years.8 The Dubbo Osteoporosis Epidemiology Study, which studied a large cohort of elderly men and women from 1989 onwards, found that after the age of 60 years, about 30% of men will suffer at least one osteoporotic fracture.9
As the prevalence of prostate cancer and osteoporosis both increase with age, many patients at diagnosis of prostate cancer may already have osteoporosis. Indeed, patients with prostate cancer about to undergo ADT (without bone metastases) have been found to have lower BMD than age-matched controls.10 The additional impact of ADT on bone metabolism has the potential to influence morbidity and mortality in prostate cancer.
ADT reduces BMD in patients with prostate cancer (Level II evidence),11 and this reduction can be demonstrated within 6 months of continuous ADT.12,13 Bone mineral density measurement by dual energy x-ray absorptiometry (DEXA) is the best means of evaluating fracture risk. It should be noted that in elderly men, the lumbar spine frequently has coexistent degenerative disease and/or calcification of paraspinal tissues,10,14 which results in pseudo-elevation of BMD with increasing age. However, quantitative computed tomography (QCT) estimates of bone density in the lumbar spine have confirmed significant loss of trabecular bone with ADT.12 Therefore, in elderly men, femoral neck BMD measurements by DEXA may be more appropriate for monitoring the impact of ADT. Estimates of bone loss vary between 2% and 8% at the lumbar spine (the higher bone loss measurement obtained with QCT), and between 1.8% and 6.5% for total hip bone loss during the first 12 months of continuous ADT. Bone loss in men on ADT appears to be more marked at sites of trabecular bone (lumbar spine, total hip, trochanter and ultradistal radius).10
Recommendations for monitoring BMD in men commencing ADT are summarised in Box 1.
Several retrospective audits have shown significantly higher fracture rates in men treated with ADT (Level III-2 evidence),11,15,16 with secondary bone metastases accounting for only 7%–16% of fractures.17,18 Of men surviving at least 5 years after diagnosis of prostate cancer, 19.4% of those receiving ADT had a fracture compared with 12.6% of those not receiving ADT (50 613 men diagnosed with prostate cancer in the Surveillance, Epidemiology and End Results Program15). The number needed to harm for the occurrence of fracture, 5 years from diagnosis, was 28 for any use of a gonadotropin-releasing hormone agonist and 16 for orchidectomy. Rates of fracture were dependent on the number of doses of gonadotropin-releasing hormone received during the 12 months after diagnosis of prostate cancer, and the age of the patient.15 Fracture rates increased significantly once five or more doses of ADT had been given within a 12-month interval, and increased further in those receiving nine or more doses of ADT in the 12 months after diagnosis, to be equivalent to rates observed in patients with orchidectomy.
The importance of vitamin D and calcium replacement in preventing osteoporosis in patients with prostate cancer should not be overlooked, particularly given an epidemiological association between vitamin D deficiency and prostate cancer risk.19 Subclinical vitamin D deficiency has been found in 63% of elderly men presenting with hip fracture, compared with 25% in a concurrent hospital-based control group (inpatients and outpatients).20 In one study examining the effect of pamidronate on BMD,12 patients in both the placebo and treatment arms were supplemented with 500 mg of calcium carbonate and 400 IU of vitamin D orally, daily. There was a significant fall in parathyroid hormone levels in both groups, although serum 25-hydroxy vitamin D levels did not increase. Vitamin D therapy alone did not improve BMD or reduce bone turnover markers in patients subsequently receiving ADT (Level II evidence). A retrospective review of 87 men receiving ADT for prostate cancer found that low serum 25-hydroxyvitamin D levels was an important risk factor for osteoporosis and spinal fracture (Level IV evidence), in addition to duration of ADT and history of alcohol excess.21 Given the potential of vitamin D deficiency to exacerbate bone loss, we have summarised recommendations for calcium and vitamin D replacement in men receiving ADT in Box 2.
A randomised study of prostate cancer patients on ADT showed that resistance exercise, consisting of supervised training at least three times per week for 30–60 minutes, improved fatigue, muscle strength and some measures of quality of life.24 However, in the 12-week exercise period there was no demonstrable effect of exercise on BMD or testosterone level.
Studies have started to look at the use of bisphosphonates to prevent bone loss in patients being treated with ADT. Two randomised controlled trials of pamidronate with concurrent ADT (Level I evidence) have shown prevention of bone loss in one study,12 and increase of lumbar spine BMD by QCT in the other.13 A randomised controlled trial of zoledronate given concurrently with ADT showed a significant increase in BMD of the hip (Level II evidence).25 By comparison, the selective oestrogen-receptor modulator, raloxifene, has also been studied in a randomised controlled trial in patients treated concurrently with ADT, and achieved an increase in BMD at the hip with 12 months therapy similar to that reported above with zoledronate (Level II evidence).22
To date, there are no studies evaluating reduction in fracture with bisphosphonates in patients with prostate cancer. However, alendronate therapy has been shown to increase BMD and reduce morphometric spinal fractures in eugonadal and hypogonadal men with osteoporosis (Level I evidence),26,27 and the effect was independent of baseline sex hormone levels.
It is clear from the literature on osteoporosis that patients with pre-existing fracture or with a minimal trauma fracture that develops during ADT should be treated with an oral bisphosphonate (in Australia, either alendronate or risedronate). Given the evidence that ADT reduces BMD and increases fracture risk, we propose that bisphosphonate therapy needs to be considered in individuals at high risk of developing fracture, either because of low pre-existing BMD (osteoporotic levels) or with significant loss of BMD while on therapy (ie, levels fall to the osteoporotic range on therapy). The choice of bisphosphonates in those without fracture will vary between individuals and will need to take into account the costs of therapy. The first choice should be an oral bisphosphonate such as alendronate or risedronate, which are currently authorised for use in male osteoporosis with fracture. Where these are not tolerated, reports have shown prevention of bone loss with doses of pamidronate varying between 60 mg at 3-monthly intervals and 90 mg at 6-monthly intervals (Level I evidence),12,13 and zoledronate at a dose of 4 mg at 3-monthly intervals (Level II evidence).25 However, pamidronate and zoledronate are not currently authorised for use in osteoporosis in Australia. Similarly, raloxifene has been shown to prevent bone loss in ADT, but is also not currently authorised in Australia for use in male osteoporosis. Choice of therapy needs to be balanced against potential complications of therapy. Pamidronate and zoledronate are much more likely to be associated with osteonecrosis of the jaw than alendronate or risedronate.28 Raloxifene is associated with a higher risk of venous thrombosis, with one episode in 24 patients treated with ADT and raloxifene having been reported.22
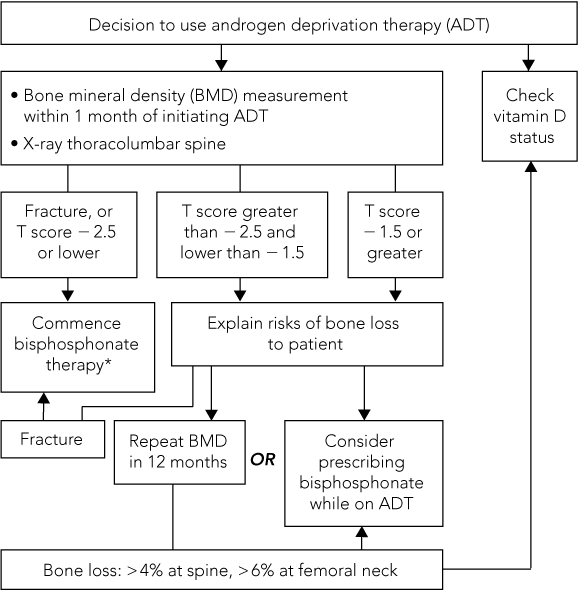
Box 3 shows a flow diagram we have developed to assist in monitoring and treatment of patients receiving ADT. Validation of our recommendations awaits prospective randomised controlled trials of reduction in fracture rates with therapy in patients treated with ADT, and not just prevention of bone loss as measured by BMD. In the interim, we encourage all doctors to monitor bone health in patients treated with ADT to minimise the impact of this therapy on bone loss, and to prevent morbidity in long-term prostate cancer survivors.
What is the effect on survival of using ADT in asymptomatic localised disease or with rising prostate-specific antigen level following radiation therapy or radical prostatectomy? There is the potential to cause reduction in survival with ADT. In patients with prostate cancer treated with chronic androgen suppression therapy, onset of skeletal fractures after diagnosis of prostate cancer has been shown to independently predict overall survival.29 Additional costs of care from use of both ADT and therapy for osteoporotic fracture need to be evaluated and survival benefit (if any) determined.
Does bisphosphonate therapy prevent fracture in patients with osteoporosis and prostate cancer with or without ADT?
How long should bisphosphonate therapy continue, and does duration depend on BMD or on the duration of ADT? Recent literature suggests durations of therapy up to a maximum of 5 years may be appropriate to avoid complications of bisphosphonate therapy, such as non-osteoporotic fractures resulting from excessive suppression of bone turnover30,31 and osteonecrosis of the jaw.28 There is one reported case of osteonecrosis of the jaw in a patient with prostate cancer treated with zoledronate.32
1 Recommendations for monitoring of bone mineral density (BMD) with androgen deprivation therapy (ADT)10,13,14
Measure baseline BMD by dual energy x-ray absorptiometry before or within 1 month of commencement of ADT or surgical orchidectomy.
Take a baseline thoracolumbar spine x-ray to screen for asymptomatic osteoporotic fracture.
Perform baseline biochemical tests, including calcium and phosphate levels, liver function tests, and levels of thyroid-stimulating hormone, 25-hydroxy vitamin D and parathyroid hormone — if there are any abnormalities refer to a specialist with an interest in osteoporosis at initiation of ADT.
Monitor BMD and biochemical parameters annually while on ADT (BMD measurement is reimbursed under Medicare for studies every 2 years, but it is recommended that BMD be repeated annually, particularly for patients with an initial T score of <−1.5 at any site).
2 Recommendations for vitamin D and calcium supplementation13,22,23
Oral calcium and vitamin D supplementation is recommended for all men diagnosed with prostate cancer whether or not they are being treated with androgen deprivation therapy.
A minimum of 500 mg daily of an oral calcium supplement should be given in addition to dietary sources of calcium.
If vitamin D levels are greater than 60 nmol/L, supplementation with at least 400 IU of vitamin D daily should be given; higher doses of 600–1000 IU/day may be required by men aged over 70 years.
Men with vitamin D deficiency (level, < 60 nmol/L) require higher doses and should be given 3000–5000 IU per day for at least 3 months, as indicated in a recent Australian position statement.23
Serum 25-hydoxy-vitamin D levels need to be monitored at 3–4-monthly intervals in patients with vitamin D deficiency requiring replacement, or annually in those who have adequate vitamin D levels.
- D Jane Holmes-Walker1
- Henry Woo2
- Howard Gurney3
- Viet T Do4
- David R Chipps5
- 1 Westmead Hospital, Sydney, NSW.
- 2 Sydney West Area Health Service, Sydney, NSW.
In 2004, Viet Do received a speaker fee for an educational afternoon for oncological sales representatives of AstraZeneca on the roles of radiotherapy (and its incorporation with hormone therapy) in the management of prostate cancer. In the same year he received educational funding from Schering to facilitate our educational activities of his patients in a workshop called "Energy for Life". Henry Woo serves on the ANZ Urology and Intercontinental Urology Advisory Board for Novartis Pharmaceuticals and has received an honorarium for participation in meetings, some of which have been held in conjunction with major international urology meetings.
- 1. Medical Research Council Prostate Cancer Working Party Investigators Group. Immediate versus deferred treatment for advanced prostatic cancer: initial results of the Medical Research Council Trial. Br J Urol 1997; 79: 235-246.
- 2. Wilt T, Nair B, MacDonald R, Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostate cancer (Cochrane review). Cochrane Database Syst Rev 2005; (4): CD003506.
- 3. Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997; 337: 295-300.
- 4. Messing EM, Manola J, Sarosdy M, et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node positive prostate cancer. N Engl J Med 1999; 341: 1781-1788.
- 5. Medicare Australia. Pharmaceutical Benefits Schedule item statistics. Available at: http://www.medicareaustralia.gov.au/statistics/dyn_pbs/forms/pbs_tab1.shtml (accessed Jan 2006).
- 6. National Health and Medical Research Council. A guide to the development, implementation, and evaluation of clinical practice guidelines. Canberra: NHMRC, 1999.
- 7. Kiebzak GM, Beinart GA, Perser K, et al. Under-treatment of osteoporosis in men with hip fracture. Arch Intern Med 2002; 162: 2217-2222.
- 8. Australian National Consensus Conference. The prevention and management of osteoporosis — consensus statement. Med J Aust 1997; 167 Suppl 1: S4-S14.
- 9. Jones G, Nguyen T, Sambrook PN, et al. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES). Osteoporosis Int 1994; 4: 277-282.
- 10. Mittan D, Lee S, Miller E, et al. Bone loss following hypogonadism in men with prostate cancer treated with GnRH analogs. J Clin Endocrinol Metab 2002; 87: 3656-3661.
- 11. Diamond TH, Higano CS, Smith MR, et al. Osteoporosis in men with prostate carcinoma receiving androgen deprivation therapy: recommendations for diagnosis and therapies. Cancer 2004; 100: 892-899.
- 12. Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med 2001; 345: 948-955.
- 13. Diamond TH, Winters J, Smith A, et al. The anti-osteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomised, placebo-controlled crossover study. Cancer 2001; 92: 1444-1450.
- 14. Jones G, Nguyen T, Sambrook P, et al. Progressive loss of bone in the femoral neck in elderly people: longitudinal findings from the Dubbo Osteoporosis Epidemiology Study. BMJ 1994; 309: 691-695.
- 15. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation therapy for prostate cancer. N Engl J Med 2005; 352: 154-164.
- 16. Oefelein MG, Ricchuiti V, Conrad W, et al. Skeletal fracture associated with androgen suppression induced osteoporosis: the clinical incidence and risk factors for patients with prostate cancer. J Urol 2001; 166: 1724-1728.
- 17. Melton LJ III, Alothman KI, Khosla S, et al. Fracture risk following bilateral orchidectomy. J Urol 2003; 169: 1747-1750.
- 18. Townsend MF, Sanders WH, Northway RO, et al. Bone fractures associated with luteinizing hormone-releasing hormone agonists used in the treatment of prostate carcinoma. Cancer 1997; 79: 545-550.
- 19. Ahonen MH, Tenkanen L, Teppo L, et al. Prostate cancer risk and pre-diagnostic serum 25 hydroxy vitamin D levels (Finland). Cancer Causes Control 2000; 11: 847-852.
- 20. Diamond T, Smerdely P, Kormas N, et al. Hip fracture in elderly men: the importance of subclinical vitamin D deficiency and hypogonadism. Med J Aust 1998; 169: 138-141.
- 21. Diamond TH, Bucci J, Kersley JH, et al. Osteoporosis and spinal fractures in men with prostate cancer: risk factors and effects of androgen deprivation therapy. J Urol 2004; 172: 529-532.
- 22. Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomised controlled trial. J Clin Endocrinol Metab 2004; 89: 3841-3846.
- 23. Vitamin D and adult bone health in Australia and New Zealand: a position statement. Working Group of the Australian and New Zealand Bone and Mineral Society, Endocrine Society of Australia and Osteoporosis Australia. Med J Aust 2005; 182: 281-285. http://www.mja.com.au/public/issues/182_06_210305/dia10848_fm.html#elementId-1089513
- 24. Segal RJ, Reid RD, Courneya KS, et al. Resistance exercise in men receiving androgen deprivation therapy for prostate cancer. J Clin Oncol 2003; 21: 1653-1659.
- 25. Smith MR, Eastham J, Gleason DM, et al. Randomised control trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol 2003; 169: 2008-2012.
- 26. Orwoll E, Ettinger M, Weiss S, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med 2000; 343: 604-610.
- 27. Ringe JD, Dorst A, Faber H, Ibach K. Alendronate treatment of established primary osteoporosis in men: 3-year results of prospective, comparative, two-arm study. Rheumatol Int 2004; 24: 110-113.
- 28. Osteonecrosis of the jaw and bisphosphonates [letters]. N Engl J Med 2005; 353: 99-102.
- 29. Oefelein MG, Ricchiutti V, Conrad W, et al. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 2002; 168: 1005-1007.
- 30. Odvina CV, Zerwekh JE, Rao DS, et al. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 2005; 90: 1294-1301.
- 31. Ott SM. Long term safety of bisphosphonates [editorial]. J Clin Endocrinol Metab 2005; 90: 1897-1899.
- 32. Olson KB, Hellie CM, Pienta KJ. Osteonecrosis of jaw in patient with hormone-refractory prostate cancer treated with zoledronic acid [review]. Urology 2005; 66: 658.





Abstract
Loss of bone mineral density with androgen deprivation therapy (ADT) for prostate cancer is well recognised, with significant loss of bone mineral density (BMD) occurring within 12 months of starting therapy.
With ADT, annual loss of BMD is about 2%–8% per year at the lumbar spine and 1.8%–6.5% at the hip; the loss appears to continue indefinitely while treatment continues, and there is no recovery after therapy is ceased.
19.4% of men surviving at least 5 years after diagnosis of prostate cancer have a fracture if treated with ADT compared with 12.6% of men not receiving ADT; this is equivalent to one additional fracture for every 28 men treated with ADT.
Vitamin D deficiency exacerbates the development of osteoporosis, so vitamin D status should be evaluated before commencing ADT in men with prostate cancer.
Treatment with bisphosphonates (zoledronate, pamidronate and alendronate) in men treated with ADT have been shown to prevent bone loss in prospective studies and to increase BMD in one randomised controlled trial; bisphosphonates have not been shown to prevent fractures in men with prostate cancer.
Further prospective trials are required to assess the efficacy and cost-effectiveness of bisphosphonates in men with prostate cancer who require treatment with ADT.
All doctors need to take an active role in monitoring bone health in patients with prostate cancer requiring ADT.