Invasive pneumococcal disease (IPD) is well recognised as an important infection in Indigenous children and adults in central and northern Australia.1-4 In recognition of this increased risk of IPD, the 23-valent pneumococcal polysaccharide vaccine (23vPPV) was made freely available to at-risk Indigenous adults in north Queensland (defined as north of a line approximately between Mt Isa and Mackay) from 1996.5
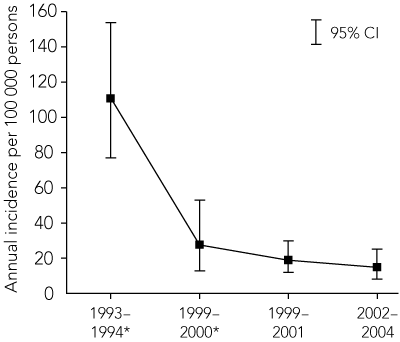
Within 4 years, the estimated annual incidence of vaccine-preventable IPD in at-risk Indigenous adults in far north Queensland (defined as north of a line between Kowanyama, Croydon and Cardwell) had declined from 111 to 28 cases per 100 000.5 However, over this time, the annual incidence of vaccine-failure IPD rose to 32 cases per 100 000. The 23vPPV was subsequently made freely available to all at-risk Indigenous adults throughout Queensland in 1998, and nationally in 1999.5
As Indigenous children also had increased risk of IPD,2,4 a recently licensed 7-valent pneumococcal conjugate vaccine (7vPCV; comprising pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F and 23F) was made freely available to all Indigenous children aged under 2 years, with a booster dose of 23vPPV (at 24 months of age in Queensland) from mid-2001.6
We report the epidemiology of IPD in Indigenous people in north Queensland over the 3 years before, and the 3 years after, 7vPCV became available free for young Indigenous children (1999–2004). We also examine whether there was any evidence of a herd immunity effect7 indirectly protecting either Indigenous children aged 5–14 years or Indigenous adults, in the 3 years following the introduction of 7vPCV for young Indigenous children.
IPD is defined by the isolation of Streptococcus pneumoniae from a usually sterile body site; it became a notifiable disease in Queensland in 1996. Diagnostic laboratories notify local Public Health Units of each case; and, in north Queensland, Tropical Public Health Unit staff then interview each patient (or the guardian), and/or the attending physician, to complete a standardised questionnaire. This incorporates details of the case, recognised IPD risk factors and prior pneumococcal vaccination.5
Invasive pneumococcal isolates are forwarded to a reference laboratory for serotyping.4
An invasive isolate was defined as “vaccine type”, if the serotype is included in the relevant (to the age of the patient) pneumococcal vaccine; all other serotypes were defined as “non-vaccine type”.
A case of IPD was defined as “vaccine-preventable” if:
It was in an Indigenous child who was age-eligible for free vaccine, and was caused by a 7vPCV vaccine type.
It was in an Indigenous adult and was caused by a 23vPPV vaccine type, and the adult was eligible for free 23vPPV8 (smoking was included among eligibility criteria for Indigenous adults from 20015), or the case occurred 5 years or longer after a dose of 23vPPV.8
A case of IPD was defined as “vaccine failure” if it was caused by a vaccine type, and the patient had documented evidence of having received a primary course of the relevant vaccine or the recommended booster (or revaccination)8 more than 2 weeks before the onset of illness.5
Only those cases of IPD that occurred in Indigenous residents of north Queensland, and were apparently acquired in the region, were included in this report.
The incidences of IPD were calculated using population denominators from the national 2001 census; there were about 53 750 Indigenous people in the region, including nearly 6900 children aged under 5 years, about 14 010 children aged 5–14 years, and nearly 5650 adults aged 50 years or older.9
The differences in the numbers of vaccine type versus non-vaccine type IPD cases in the two 3-year periods were tested with the χ2 test with Yates correction; the confidence intervals for the incidence rates were calculated using tabulated factors.10
Ethical approval was not necessary for this study as surveillance of communicable diseases of public health importance is required by law.
The annual incidence of IPD in Indigenous children and adults in 1999–2001 and 2002–2004 is shown in Box 1.
In the 3 years after the introduction of 7vPCV, there was a 54% decline (95% CI, 47%–63%) in IPD in Indigenous children aged under 5 years and a 58% decline (95% CI, 47%–75%) in those aged 5–14 years. There was also a 37% decline (95% CI, 31%–42%) in IPD caused by 23vPPV serotypes and a 43% decline (95% CI, 34%–53%) in 23vPPV vaccine failures in Indigenous adults.
There were 72 cases of IPD in Indigenous children aged , 15 years in north Queensland between 1999 and 2004.
Of the 72 cases, 51 (71%) were in children aged under 5 years (Box 2). Thirty-two of the 51 children had pneumonia (63%); 12 bacteraemia (24%); five pneumococcal meningitis (10%) with one death (serotype 6A); one had septic arthritis; and one, submandibular abscess.
Forty-six of the 51 isolates were serotyped, and 27 (59%) were non-vaccine type, with similar numbers in 1999–2001 and 2002–2004 (Box 2). The non-vaccine types were distributed among 13 serotypes, with types 1, 19A, 6A and 33F being the most common (Box 3).
Nineteen isolates were vaccine type — 17 (89%) in 1999–2001. There was a significant decline in vaccine-type IPD in Indigenous children aged under 5 years following the introduction of 7vPCV (Yates corrected χ2 = 5.57, P = 0.02). The two vaccine-preventable cases in 2002–2004 were both in 3-year-old children, one partly vaccinated and the other unvaccinated.
Eleven cases of IPD occurred in Indigenous children aged 2–14 years in 2002–2004, seven caused by a 23vPPV vaccine type. Six of these seven children had never received the recommended 23vPPV booster dose (scheduled at 24 months of age in Queensland). There was one 23vPPV vaccine failure (serotype 12F), in a 2.5-year-old child who had received the vaccine at 21 months of age.
There were 21 IPD cases in Indigenous children aged 5–14 years — 15 (71%) in 1999–2001 (Box 2). Eight of these 21 cases (38%) were caused by 7vPCV vaccine-type pneumococci — six in 1999–2001, and two in 2002–2004.
There were 108 cases of IPD in Indigenous adults aged ≥ 15 years in north Queensland between 1999 and 2004 (Box 2). Of these, 89 (82%) occurred in adults 15–49 years of age, with similar numbers in 1999–2001 and 2002–2004. Seventy-one patients had pneumonia (80%), 11 bacteraemia (12%) and four meningitis; three died.
Nineteen cases were in adults aged ≥ 50 years, with 12 (63%) in 1999–2001.
All but one of the isolates was serotyped: 28 (26%) were 23vPPV non-vaccine type (nine [32%] in 1999–2001), while 79 were vaccine type (48 [61%] in 1999–2001). There was significantly more vaccine-type IPD in Indigenous adults aged ≥ 15 years in 1999–2001 compared with 2002–2004 (Yates corrected χ2 = 5.7, P = 0.02).
Distribution of vaccine-type isolates is shown in Box 4. Twenty-two isolates belonged to the seven serotypes included in both 23vPPV and 7vPCV, with 16 (73%) in 1999–2001. There was a non-significant decline in IPD caused by these seven serotypes compared with all other serotypes in Indigenous adults between 1999–2001 and 2002–2004 (Yates corrected χ2 = 3.28, P = 0.07).
Nine (11%) of the 79 patients with vaccine-type IPD had no identified risk factor for IPD and were therefore not eligible for 23vPPV. Thirty-four of the remaining cases (49%) were classified as vaccine-preventable, with seven of these in people who had failed to receive a first 5-year revaccination. The other 36 (51%) were vaccine failures.
The annual incidence of IPD in Indigenous children aged under 5 years declined by 54% (95% CI, 47%–63%) in the 3 years after the introduction of 7vPCV. Virtually all the decline was attributable to a decline in vaccine-type IPD, providing further evidence that 7vPCV is very effective in preventing vaccine-type IPD, even in disadvantaged children.11 However, as almost all (87%) of the IPD that occurred in these children in 2002–2004 was non-vaccine type, it is unlikely that the incidence of IPD in young Indigenous children will fall much further with the current 7-valent vaccine.
As documented previously,4 the predominant (63%) clinical IPD syndrome in Indigenous children under 5 years of age was pneumonia. Undoubtedly, 7vPCV has prevented much bacteraemic pneumonia in vaccinated Indigenous children, but it may have also prevented a substantial burden of non-bacteraemic pneumonia in these children. In a clinical trial in the United States, 7vPCV reduced clinically diagnosed pneumonia with positive radiographic changes by 20.5%, with the greatest reduction (32%) in the first year of life.12 The vast majority of the patients with pneumonia in that study did not have pneumococcal bacteraemia.12 Similarly, a 9-valent pneumococcal vaccine (which also includes serotypes 1 and 5) was shown to reduce radiologically confirmed pneumonia by 20% in HIV-negative children in South Africa, after a three-dose primary series in infancy and no booster.13
We also observed a 58% decline (95% CI, 47%–75%) in the incidence of IPD in Indigenous children aged 5–14 years from 1999–2001 to 2002–2004. Although the small number of cases precludes any certainty, it is quite possible that a herd immunity effect, with vaccinated young Indigenous children indirectly protecting their older siblings,7 is involved. A recent decline in IPD in people aged 5–17 years, presumably a similar older sibling herd immunity effect, has recently been reported from the United States.14
It is of interest that serotypes 6A and 19A were the leading non-vaccine types causing IPD in Indigenous children under 5 years of age in 2002–2004, as these two serotypes are closely related to the vaccine serotypes 6B and 19F, respectively. All six young Indigenous children with IPD caused by serotype 6A or 19A had received some 7vPCV, with four having completed a three-dose primary series (data not shown). Post-licensure surveillance has raised concerns about cases of serotype 19A IPD occurring in vaccinated children in the US,15,16 and an epidemiological study was not able to demonstrate any cross-protection against 19A.17 Although it did demonstrate cross-protection against serotype 6A,17 laboratory studies suggest that this cross-protection does not have optimal functional activity,18 possibly explaining why serotype 6A has persisted among vaccinated Indigenous children in north Queensland.
There was also a significant decline in vaccine-type IPD in Indigenous adults between 1999–2001 and 2002–2004, but no significant decline in IPD caused by the 7vPCV serotypes, suggesting that there was no indirect herd immunity effect following the vaccination of Indigenous children.
However, there was clearly an outbreak of IPD caused by serotype 1 affecting Indigenous people in north Queensland in 1999–2001 (Boxes 3 and 4). Of the 24 serotype 1 isolates identified between 1999 and 2004, 19 occurred in 1999–2001, with 15 of these in Indigenous people. Outbreaks of serotype 1 disease have been previously reported among disadvantaged communities, including Aboriginal Australians.19,20
Excluding all the serotype 1 cases, there was then a significant reduction in IPD caused by serotypes included in both 23vPPV and 7vPCV compared with all other serotypes in Indigenous adults between 1999–2001 and 2002–2004 (Yates corrected χ2 = 4.50, P = 0.03). This suggests that the serotype 1 outbreak obscured an indirect herd immunity effect, which protected Indigenous adults following the introduction of 7vPCV for Indigenous children from the latter part of 2001. A similar herd immunity effect protecting adults from IPD was observed following the introduction of 7vPCV for children in the US.15,17
Although the decline in vaccine-preventable IPD in Indigenous adults between the two periods was not significant, there has been a continued decline since 23vPPV was first used in Indigenous adults in the region from 1996 (Box 5). The continued downward trend is very encouraging, and suggests that there is not only high vaccine coverage among at-risk Indigenous adults in the region,5 but also that the vaccine is effective in this population. If the latter is correct, it is in marked contrast to the finding that 23vPPV was not effective in preventing vaccine-type IPD in a population of Native American adults in the US.21
Also encouraging was the significant decline in IPD vaccine failures in Indigenous adults between the two periods. This may reflect the reduced transmission of serotype 1 and the 7vPCV vaccine types, but it may also be a consequence of revaccination with 23vPPV, as many of the Indigenous adults vaccinated for the first time from 1998 onwards would have been eligible for revaccination from 2002. However, a relative immune hyporesponsiveness follows revaccination, and there are very limited data on the protection following revaccination with 23vPPV.22 Regardless, it does not appear that revaccination with 23vPPV is associated with an increased risk of adverse events significant enough to require medical review.23
1 Average annual incidence (95% CI) of invasive pneumococcal disease (IPD) per 100 000 Indigenous people in north Queensland before and after introduction of the 7-valent vaccine in 2001
|
1999–2001 |
2002–2004 |
|||||||||||||
Indigenous children |
|
|
|||||||||||||
Age < 5 years |
170 (118–236) |
78* (44–126) |
|||||||||||||
Age, 5–14 years |
36 (20–59) |
15* (5–31) |
|||||||||||||
Indigenous adults |
|
|
|||||||||||||
Age ≥ 50 years |
71 (37–124) |
41 (6–85) |
|||||||||||||
Age, 15–49 years |
56 (41–75) |
53 (38–71) |
|||||||||||||
Vaccine-type IPD† |
49 (36–65) |
31*(21–45) |
|||||||||||||
Vaccine preventable |
19 (12–30) |
15 (8–25) |
|||||||||||||
Vaccine failure |
23 (15–35) |
13* (7–23) |
|||||||||||||
* These point estimates are below the 95% CIs of the preceding 3 years. † Vaccine-type IPD = IPD caused by serotypes included in the 23-valent pneumococcal polysaccharide vaccine. |
|||||||||||||||
2 Cases of invasive pneumococcal disease (IPD) in Indigenous people in north Queensland before and after introduction of the 7-valent vaccine in 2001
|
All isolates |
Non-vaccine type* |
Vaccine type* |
||||||||||||
|
Total |
1999–2001 |
2002–2004 |
Total |
1999–2001 |
2002–2004 |
Total |
1999–2001 |
2002–2004 |
||||||
Children |
72 |
50 |
22 |
40 |
23 |
17 |
27 |
23 |
4 |
||||||
Age < 5 years |
51 |
35 |
16 |
27† |
14 |
13 |
19† |
17 |
2‡ |
||||||
Age, 5–14 years |
21 |
15 |
6 |
13 |
9 |
4 |
8 |
6 |
2 |
||||||
Adults |
108 |
58 |
50 |
28§ |
9 |
19 |
79§ |
48 |
31 |
||||||
Age, 15–49 years |
89 |
46 |
43 |
23 |
7 |
16 |
65 |
38 |
27 |
||||||
Age ≥ 50 years |
19 |
12 |
7 |
5 |
2 |
3 |
14 |
10 |
4 |
||||||
* Vaccine-type IPD = IPD caused by serotypes included in the 7-valent pneumococcal conjugate vaccine in the case of children <15 years, and the 23-valent pneumococcal polysaccharide vaccine in the case of adults ≥ 15 years. † 46 of the 51 isolates were serotyped. ‡ Both cases were classed as vaccine-preventable. Both children were aged 3 years: one was partly vaccinated and the other unvaccinated. § 107 of the 108 isolates were serotyped. |
|||||||||||||||
3 Distribution of non-vaccine serotypes* among invasive pneumococci isolated from children aged < 5 years in north Queensland
Serotype |
1999–2001 |
2002–2004 |
Total |
||||||||||||
1 |
6 |
0 |
6 |
||||||||||||
5 |
1 |
0 |
1 |
||||||||||||
6A |
1 |
3 |
4 |
||||||||||||
7F |
0 |
1 |
1 |
||||||||||||
8 |
0 |
1 |
1 |
||||||||||||
10A |
0 |
1 |
1 |
||||||||||||
12F |
0 |
1 |
1 |
||||||||||||
13 |
1 |
0 |
1 |
||||||||||||
16F |
0 |
1 |
1 |
||||||||||||
17F |
1 |
0 |
1 |
||||||||||||
18B |
1 |
0 |
1 |
||||||||||||
19A |
2 |
3 |
5 |
||||||||||||
33F |
1 |
2 |
3 |
||||||||||||
Total |
14 |
13 |
27 |
||||||||||||
* Serotypes not included in the 7-valent pneumococcal conjugate vaccine. |
|||||||||||||||
4 Distribution of vaccine serotypes among invasive pneumococci isolated from Indigenous adults ≥ 15 years of age in north Queensland
Serotype |
1999–2001 |
2002–2004 |
Change |
||||||||||||
Serotypes in both vaccines* |
|
||||||||||||||
4 |
4 |
1 |
− 3 |
||||||||||||
6B |
1 |
0 |
− 1 |
||||||||||||
9V |
3 |
2 |
− 1 |
||||||||||||
14 |
2 |
2 |
0 |
||||||||||||
18C |
2 |
1 |
− 1 |
||||||||||||
19F |
3 |
0 |
− 3 |
||||||||||||
23F |
1 |
0 |
− 1 |
||||||||||||
Total |
16 |
6 |
− 10 |
||||||||||||
23vPPV-only serotypes |
|
||||||||||||||
1 |
7 |
1 |
− 6 |
||||||||||||
3 |
3 |
3 |
0 |
||||||||||||
5 |
3 |
0 |
− 3 |
||||||||||||
7F |
7 |
7 |
0 |
||||||||||||
8 |
2 |
5 |
+3 |
||||||||||||
10A |
1 |
1 |
0 |
||||||||||||
11A |
2 |
1 |
− 1 |
||||||||||||
12F |
2 |
2 |
0 |
||||||||||||
19A |
4 |
2 |
− 2 |
||||||||||||
22F |
0 |
3 |
+3 |
||||||||||||
33F |
1 |
0 |
− 1 |
||||||||||||
Total |
32 |
25 |
− 7 |
||||||||||||
All vaccine serotypes |
48 |
31 |
−17 |
||||||||||||
* Serotypes included in both the 7-valent (7vPCV) and 23-valent (23vPPV) vaccines. |
|||||||||||||||
Received 22 June 2005, accepted 2 November 2005
- Jeffrey N Hanna1
- Jan L Humphreys2
- Denise M Murphy3
- 1 Tropical Public Health Unit Network, Queensland Health, Cairns, QLD.
- 2 Tropical Public Health Unit Network, Queensland Health, Aitkenvale, QLD.
- 3 Pneumococcal Reference Laboratory, Queensland Health Scientific Services, Coopers Plains, QLD.
We thank all laboratory and clinical staff involved in the diagnosis and management of the patients with invasive pneumococcal disease included in this report, and those in the Tropical Public Health Unit Network who undertook follow-up and collected information.
Jeffrey Hanna has received honoraria and travel grants from vaccine manufacturers, including Merck Sharp and Dohme and Wyeth.
- 1. Torzillo PJ, Hanna JN, Morey F, et al. Invasive pneumococcal disease in central Australia. Med J Aust 1995; 162: 182-186.
- 2. Krause VL, Reid SJC, Merianos A. Invasive pneumococcal disease in the Northern Territory of Australia, 1994-1998. Med J Aust 2000; 173 Suppl: S27-S31.
- 3. Hanna JN, Gratten M, Tiley SM, et al. Pneumococcal vaccination: an important strategy to prevent pneumonia in Aboriginal and Torres Strait Island adults. Aust N Z J Public Health 1997; 21: 281-285.
- 4. Fagan RL, Hanna JN, Messer RD, et al. The epidemiology of invasive pneumococcal disease in children in Far North Queensland. J Paediatr Child Health 2001; 37: 571-575.
- 5. Hanna JN, Young DM, Brookes DL, et al. The initial coverage and impact of the pneumococcal and influenza vaccination program for at-risk Indigenous adults in Far North Queensland. Aust N Z J Public Health 2001; 25: 543-546.
- 6. Hanna JN, Bullen RC, Ziegler CL, et al. An assessment of the implementation of the pneumococcal conjugate vaccination program for Aboriginal and Torres Strait infants in north Queensland. Commun Dis Intell 2003; 27: 262-266.
- 7. O’Brien KL, Dagan R. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine 2003; 21: 1815-1825.
- 8. National Health and Medical Research Council. Pneumococcal infections. In: The Australian Immunisation Handbook. 8th ed. Canberra: Commonwealth of Australia, 2003: 220-234.
- 9. Australian Bureau of Statistics. 2001 Census of Population and Housing (first release, Aboriginal and Torres Strait Island profile by statistical local area). Canberra: ABS, 2002.
- 10. Bailar JC, Ederer F. Significance factors for the ratio of a Poisson variable to its expectation. Biometrics 1964; 20: 639-643.
- 11. O’Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 2003; 362: 355-361.
- 12. Black SB, Shinefield HR, Ling S, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr Infect Dis J 2002; 21: 810-815.
- 13. Klugman KP, Madhi SA, Huebner RE, et al. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med 2003; 349: 1341-1348.
- 14. Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease – United States, 1998-2003. MMWR Morb Mortal Wkly Rep 2005; 54: 893-897.
- 15. Black S, Shinefield H, Baxter R, et al. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J 2004; 23: 485-489.
- 16. Kaplan SL, Mason EO, Wald ER, et al. Decrease of invasive pneumococcal infections in children among 8 children’s hospitals in the United States after the introduction of the 7-valent pneumococcal conjugate vaccine. Pediatrics 2004; 113: 443-449.
- 17. Whitney CG, Farley MM, Hadler J, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med 2003; 348: 1737-1746.
- 18. Väkeväinen M, Eklund C, Eskola J, Käyhty H. Cross-reactivity of antibodies to type 6B and 6A polysaccharides of Streptococcus pneumoniae, evoked by pneumococcal conjugate vaccines, in infants. J Infect Dis 2001; 184: 789-793.
- 19. Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis 2005; 5: 83-93.
- 20. Gratten M, Morey F, Dixon J, et al. An outbreak of serotype 1 Streptococcus pneumoniae infection in central Australia. Med J Aust 1993; 158: 340-342.
- 21. Benin AL, O’Brien KL, Watt JP, et al. Effectiveness of the 23-valent polysaccharide vaccine against invasive pneumococcal disease in Navajo adults. J Infect Dis 2003; 188: 81-89.
- 22. Whitney CG, Schaffner W, Butler JC. Rethinking recommendations for use of pneumococcal vaccines in adults. Clin Infect Dis 2001; 33: 662-675.
- 23. Walker FJ, Singleton RJ, Bulkow LR, et al. Reactions after 3 or more doses of pneumococcal polysaccharide vaccine in adults in Alaska. Clin Infect Dis 2005; 40: 1730-1735.





Abstract
Objective: To describe the epidemiology of invasive pneumococcal disease (IPD), and the impact of pneumococcal vaccines on IPD, in Indigenous people in north Queensland.
Setting: North Queensland, 1999–2004; there are about 53 750 Indigenous people in the region, including nearly 6900 children < 5 years and nearly 5650 adults ≥ 50 years.
Main outcome measures: Incidences of IPD in Indigenous children and in Indigenous adults compared between the 3 years before and after the introduction of a 7-valent pneumococcal conjugate vaccine (7vPCV) (1999–2001 versus 2002–2004).
Results: Estimated annual incidence of IPD in Indigenous children < 5 years of age declined from 170 to 78 cases per 100 000 in the 3 years following the introduction of 7vPCV in 2001. The annual incidence of vaccine-preventable IPD in Indigenous adults had declined by 86% since a 23-valent pneumococcal polysaccharide vaccine (23vPPV) was introduced to the region in 1996, to 15 cases per 100 000 (95% CI, 8–25) in 2002–2004.
Conclusion: Although there was a rapid decline in IPD in young Indigenous children, it is unlikely that the incidence will fall much further with the current 7-valent vaccine. There was a suggestion that vaccinating Indigenous children indirectly protected those aged 5–14 years and Indigenous adults ≥15 years of age. Incidence of IPD in Indigenous adults in 2002–2004 was the lowest on record in the region.