An infant, born at 35 weeks’ gestation to a woman who sniffed petrol, had a cord blood lead level eight times the accepted limit. Treatment with oral dimercaptosuccinic acid promptly reduced his blood lead levels. To our knowledge, this is the first reported case of congenital lead poisoning secondary to maternal petrol sniffing. We suggest that at-risk pregnancies should be identified, cord blood lead levels tested, and chelation therapy and developmental follow-up offered to affected infants.
A 27-year-old Indigenous woman, who had sniffed petrol since childhood, presented for antenatal care during her first pregnancy. At the age of 14 years, she had severe lead encephalopathy that led to chronic neurological deficits, including permanent ataxia and memory impairment. Her serum lead levels at 8 and 35 weeks’ gestation were raised at 1.48 and 2.21 μmol/L, respectively (recommended level, ≤ 0.48 μmol/L1).
At 35 weeks’ gestation, she went into spontaneous labour and gave birth vaginally to a boy. The infant’s Apgar score was 9 at both 1 and 5 minutes, and birth weight was 2280 g. He was admitted to the special care nursery because of prematurity, and required nasogastric tube feeding because of poor sucking and general sleepiness.
On Day 6, the infant developed temperature instability and diarrhoea, with associated dehydration and metabolic acidosis (Box 1). He was treated with 48 hours of intravenous antibiotics and intravenous fluids. No pathogen was grown from blood cultures, urine or stool.
Cord blood lead levels were available on Day 10 and were raised at 3.98 μmol/L. No signs of lead encephalopathy were found on examination: muscle tone and reflexes were normal, and there were no signs of seizure activity. Treatment was begun with oral succimer (dimercaptosuccinic acid [DMSA]) on Day 11, at a dose of 10 mg/kg three times daily for 5 days, followed by 10 mg/kg twice daily for 14 days. No adverse effects of chelation therapy (such as vomiting, diarrhoea, fever, rash, or elevated serum transaminase levels) were noted. The infant’s blood lead levels decreased with succimer treatment (Box 2), liver function results remained in the reference range, and his alertness and feeding improved by the 5th day of treatment.
At 28 days of age, the infant was feeding well on formula and weighed 3160 g. He was discharged into foster care, and succimer therapy was ceased on Day 35. His growth and development were monitored. At the age of 12 months, he had global developmental delay, with an overall Denver Developmental level of 6–7 months.2 His blood lead levels at 4, 6 and 9 months of age were at least twice the upper acceptable limit (Box 2), although not at the level at which chelation therapy is recommended (> 2.16 μmol/L).3
Lead exposure in early childhood has long been known to have significant adverse effects on cognitive development.4,5 The National Health and Medical Research Council recommends that lead levels for all Australians be less than 0.48 μmol/L (10 μg/dL).1 However, intellectual impairment is seen in children with lower blood lead levels, and there may be no safe lower limit.6 Chelation therapy is recommended for any child with blood lead levels of 2.16 μmol/L and over.3 Although chelation therapy in early childhood lowers blood levels, recent studies have not shown significant improvement in cognitive and behavioural measurements compared with untreated children.3,4,7
There are a few case reports of neonatal lead intoxication that occurred from maternal exposure to lead through pica, home renovation, or use of contaminated herbal medications.8-10 Petrol sniffing is a form of substance misuse that is widespread in some Indigenous communities in Australia, and is associated with elevated serum lead levels.11 Changing the available petrol to an unleaded form should theoretically lessen the burden of lead toxicity in petrol sniffers. However, the aromatic hydrocarbons in both forms of petrol still cause considerable acute neurotoxicity,12 and petrol sniffers appear to prefer leaded petrol.13
Infants born to women who sniff petrol are more likely to have a birth weight < 2500 g than the infants of non-sniffers, and to require admission to a neonatal nursery for care, although this may be related to other associated lifestyle factors such as smoking and alcohol use.14
Lead freely crosses the placenta and is found in cord blood, amniotic fluid and fetal tissues.8 Cord blood lead levels are often higher than maternal blood levels,8,10 which might indicate preferential placental transfer,8 or be related to the higher neonatal haematocrit.9 In our patient, the cord blood lead level (3.98 μmol/L) was higher than levels in maternal blood collected 2 days before delivery (2.21 μmol/L). Reported effects of congenital lead exposure include intrauterine growth restriction, long-term cognitive problems, and radiographic abnormalities, such as increased bone density at the metaphyses.8,9 Acute neurotoxicity manifested as encephalopathy and peripheral neuropathy has also been described.10
Chelating agents used in the management of lead poisoning include intravenous sodium calcium edetate (CaNa2EDTA) and oral succimer (DMSA).3 Oral succimer is as effective as parenteral CaNa2EDTA.15 It has been previously reported that oral DMSA therapy has not been effective in the chelation of lead in newborns,10 although it is a proven and safe therapy in older children and adults.4,15 In our infant patient, blood lead level decreased spontaneously by 11% (0.45 μmol/L) during the first 10 days of life. After chelation therapy began, serum lead levels fell by 55% in 9 days. No drug-related side effects were noted in the infant.16
This infant did not show clinical signs of acute encephalopathy. His feeding and alertness improved after the start of chelation, but it is uncertain if this was due to falling lead levels or to the normal maturation of the premature infant. After the completion of therapy, serial blood lead levels showed a slow decline, but remained above the recommended level. This was most likely due to a slow release of bound lead from bone,8 but could also have resulted from ongoing exposure to environmental lead. As in similar cases, the infant had global developmental delay at 12 months of age despite chelation therapy.10 Factors other than lead, including malnutrition, recurrent infections and socioeconomic deprivation, might also have contributed to a poor developmental outcome.
Petrol sniffing is a significant problem in some Indigenous communities, and it is likely that more infants will be born with lead exposure. Ideally, women who sniff petrol should be identified early in pregnancy. Interventions that might reduce the burden of fetal lead exposure include stopping further petrol sniffing, and giving calcium supplements to the mother to reduce bone resorption, as mobilisation of lead from maternal bone stores is a significant source of fetal lead exposure.17 Chelation agents are contraindicated in pregnancy, and are used only if maternal lead poisoning is life-threatening.8
Cord blood lead levels should be tested in at-risk infants, and chelation therapy given if levels are levels are over 2.16 μmol/L.3 Affected children are at high risk of neurodevelopmental delay. Follow-up and formal developmental assessment is made difficult in remote Indigenous communities by communication barriers, social and economic deprivation, and geographic isolation. Every effort should be made to offer early intervention services, as well as measures to optimise nutrition and general health.
1 Laboratory results for a neonate with lead poisoning and suspected sepsis
|
Reference range* |
Day 4 |
Day 5 |
||||||||||||
|
10:00 |
13:00 |
19:35 |
07:30 |
|||||||||||
Serum levels |
|
|
|
|
|
||||||||||
Sodium (mmol/L) |
133–146 |
140 |
140 |
141.6 |
140 |
||||||||||
Potassium (mmol/L) |
4.6–6.7 |
4.9 |
4.5 |
4.7 |
4.8 |
||||||||||
Urea (mmol/L) |
1.1–9.1 |
3.1 |
2.8 |
|
1.3 |
||||||||||
Creatinine (μmol/L) |
56–146 |
65 |
65 |
|
43 |
||||||||||
HCO3 (mmol/L) |
18–25 |
12.5 |
12.8 |
14.5 |
21.3 |
||||||||||
Venous pH |
7.35–7.45 |
7.17 |
7.19 |
7.26 |
7.35 |
||||||||||
Base excess (mmol/L) |
− 4 to + 3 |
− 17.6 |
− 17.3 |
− 14.7 |
− 3.8 |
||||||||||
Haemoglobin (g/L) |
150–170 |
179 |
|
|
|
||||||||||
Blood counts (× 109/L)† |
|
|
|
|
|
||||||||||
White cells |
5.0–21.0 |
7.7 |
|
|
|
||||||||||
Neutrophils |
1.5–10.0 |
1.6 |
|
|
|
||||||||||
Platelets |
150–350 |
217 |
|
|
|
||||||||||
* For preterm infant. † Blood film appeared normal for a preterm infant, with no basophilic stippling of red blood cells. |
|||||||||||||||
2 Serial blood lead levels in an infant with lead poisoning
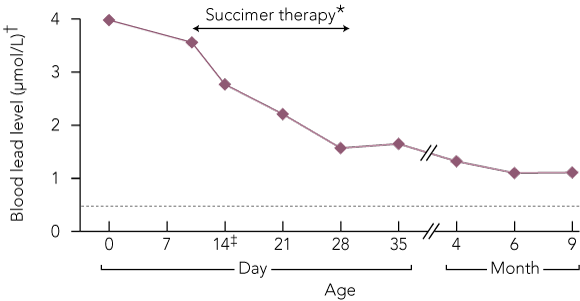
* Chelation treatment with dimercaptosuccinic acid was given from Days 11 to 29 at a dose of 10 mg/kg, initially three times daily, reducing to twice daily from Day 16. † Maximum acceptable blood level of lead = 0.48 μmol/L. ‡ Urine lead level was raised at 0.82 μmol/L (maximum acceptable level = 0.02 μmol/L).
- Suzanna T Powell1
- Srinivas Bolisetty2
- Gavin R Wheaton3
- 1 Tamworth Base Hospital, Tamworth, NSW.
- 2 Royal Hospital for Women, Sydney, NSW.
- 3 Women’s and Children’s Hospital, Adelaide, SA.
- 1. National Health and Medical Research Council. Revision of the Australian guidelines for lead in blood and lead in ambient air Canberra: NHMRC, 1993.
- 2. Frankenburg WK, Dodds J, Archer P, et al. The DENVER II technical manual. Denver, Colo: Denver Developmental Materials Inc, 1996.
- 3. Treatment guidelines for lead exposure in children. American Academy of Pediatrics Committee on Drugs. Pediatrics 1995; 96 (1 Pt 1):155-160.
- 4. Dietrich KN, Ware JH, Salganik M, et al. Effect of chelation therapy on the neuropsychological and behavioral development of lead-exposed children after school entry. Pediatrics 2004; 114: 19-26.
- 5. Pocock SJ, Smith M, Baghurst P. Environmental lead and children’s intelligence: a systematic review of the epidemiological evidence. BMJ 1994; 309: 1189-1197.
- 6. Canfield RL, Henderson CR, Cory-Slechta DA, et al. Intellectual impairment in children with blood lead concentrations below 10 microg per deciliter. N Engl J Med 2003; 348: 1517-1526.
- 7. Tong S, Baghurst PA, Sawyer MG, et al. Declining blood lead levels and changes in cognitive function during childhood. The Port Pirie Cohort Study. JAMA 1998; 280: 1915-1919.
- 8. Gardella C. Lead exposure in pregnancy: a review of the literature and argument for routine prenatal screening. Obstet Gynecol Surv 2001; 56: 231-238.
- 9. Shannon M. Severe lead poisoning in pregnancy. Ambul Pediatr 2003; 3: 37-39.
- 10. Tait PA, Vora A, James S, et al. Severe congenital lead poisoning in a preterm infant due to a herbal remedy. Med J Aust 2002; 177: 193-195. <MJA full text>
- 11. Goodheart RS, Dunne JW. Petrol sniffer’s encephalopathy. Med J Aust 1994; 160: 178-181.
- 12. Goodheart RS, Dunne JW. Petrol sniffer’s encephalopathy. Med J Aust 1994; 160: 178-181.
- 13. Fortenberry, JD. Gasoline sniffing. Am J Med 1985; 79: 740-744.
- 14. Dodd J. Petrol sniffing in a pregnant Aboriginal population: a review of maternal and neonatal outcomes. Aust N Z J Obstet Gynaecol 2001; 41: 420-423.
- 15. Graziano JH, Lolacono NJ, Moulton T, et al. Controlled study of meso-2,3-dimercaptosuccinic acid for the management of childhood lead intoxication. J Pediatr 1992; 120: 133-139.
- 16. Product information. Chemet (succimer). Deerfield, Ill: Ovation Pharmaceuticals Inc, 2003.
- 17. Gulson BL, Mizon KJ, Korsch MJ, et al. Mobilization of lead from human bone tissue during pregnancy and lactation — a summary of long-term research. Sci Total Environ 2003; 303: 79-104.




