It has long been observed that rheumatic fever (RF) appears to occur only in susceptible individuals. This is based on observations of a familial association,1 controlled studies2,3 and the fact that only a limited proportion of individuals exposed to rheumatogenic Group A streptococci subsequently develop RF.4,5
Various potential markers of susceptibility to RF have been investigated, including blood groups and human leukocyte antigen (HLA) subtypes, but findings have often been inconsistent.6-8 A non-HLA B-cell antigen known as D8/17 has been investigated in several population groups. A study of American and Caribbean people showed that, in healthy individuals, a mean of 6.12% of B cells were positive for D8/17, compared with 33.5% in patients with a past history of RF (P < 0.0001). Intermediate levels were seen in unaffected first-degree relatives of index cases, suggesting that D8/17 might be an inherited factor.9 Subsequently, D8/17 was found to be highly sensitive as a marker for previous RF in the United States, Mexico, Russia, Chile, and Israel.10,11 However, in north India it was less sensitive than other, locally derived B-cell markers.12-14
The World Health Organization recognises an urgent need to develop new strategies to control RF.15 The ability to identify a subgroup of individuals at increased risk of RF could help direct primary prevention programs.15
The D8/17 non-HLA B-cell antigen has not been studied in Australia, where Aboriginal people have among the world’s highest prevalence of rheumatic heart disease (RHD).16 Populations with the highest rates of RF and RHD often live far from laboratories with the technology to perform D8/17 assays. Assays for D8/17 expression described to date usually require testing of fresh whole blood within 1 or 2 hours of sample collection, which has made it difficult to assess D8/17 expression in people living in remote settings. We aimed to study the expression of the B-cell marker D8/17 in Australians with a past history of RF or RHD, their relatives and healthy people. We also studied some technical modifications to the D8/17 assay that might allow it to be used for people living in remote settings.
Participants were enrolled in three centres:
a remote Aboriginal community in the Northern Territory;
a regional tertiary referral hospital; and
a tertiary paediatric centre in Melbourne.
Patients with acute RF, past RF (with or without RHD), healthy relatives of these patients and healthy volunteers unrelated to the patients were recruited. The diagnosis of previous RF was confirmed from medical notes. In most cases the Jones criteria17 were supplemented with an echocardiogram report consistent with RHD. The absence of a history of RF or RHD in relatives and controls was documented prospectively by interview with the individual, review of medical notes and cardiac auscultation. Relationship status was determined by a detailed discussion in the local language.
Blood was collected in lithium heparin vacutainers (Becton Dickinson Australia, Sydney, NSW). Patients’ diagnostic categories were concealed from laboratory staff. The flow cytometry assay for D8/17 as described by Chapman et al was used.18 In brief, either 20 μL of mouse anti-D8/17 monoclonal antibody (provided by Professor J Zabriskie, Rockefeller University, New York, USA) or 5 μL of mouse IgM, lambda (isotype control; Sigma, Sigma-Aldrich, Sydney, NSW) was added to 300 μL of whole blood. After 40 minutes’ incubation at 4°C, the samples were washed with phosphate buffered saline (PBS). Fluorescein-5-isothiocyanate (FITC)-labelled goat anti-mouse IgM (μ) antibody (Caltag, Burlingame, Calif, USA) and phycoerythrin-labelled anti-CD19 (Becton Dickinson Australia, Sydney, NSW) were added. After 20 minutes’ incubation at room temperature, red cells were lysed with ammonium chloride lysis solution (150 mM ammonium chloride, 10 mM potassium bicarbonate, 1 mM tetrasodium EDTA) for 15 minutes. Cells were centrifuged and washed with PBS before resuspension in PBS for analysis by flow cytometry.
Based on published values for D8/17,9 a sample size of only two patients per group was needed to differentiate mean B-cell D8/17 expression between patients with RF or RHD and control patients, and only four patients per group were needed to differentiate between patients with RF or RHD and unaffected family members (power, 90%; two-sided α, 0.05).
One hundred and six individuals were enrolled; all but 19 were Aboriginal (Box 1). Participants included 42 males and 64 females; the proportions of males and females did not differ significantly between the groups.
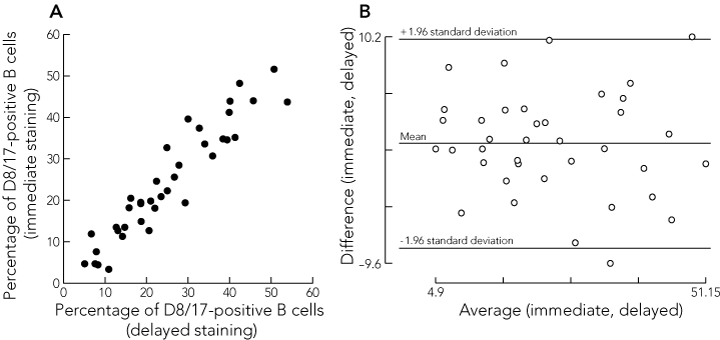
Box 4A shows a good correlation between the results for the D8/17 analysis of blood stained immediately and that stained with a delay of about half a day (mean, 0.55 days; SD, 0.2) for 38 sample pairs (correlation coefficient [r] = 0.94). A Bland–Altman plot (Box 4B) confirmed that differences between the two methods were not related to the level of D8/17 positivity. There was poor correlation between the results of the D8/17 assay obtained from analysis of fresh whole blood compared with frozen PBMC, in 19 sample pairs (r = 0.32).
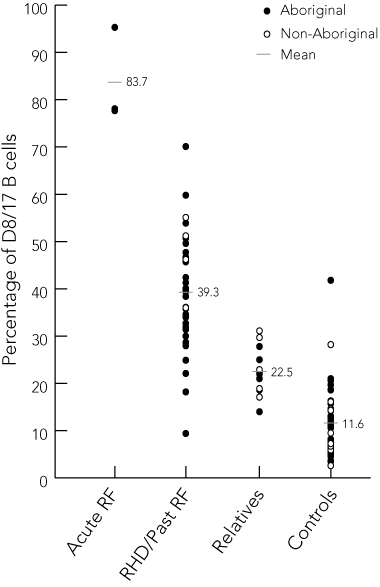
Our findings indicate that the B-cell antigen D8/17 is a sensitive and specific marker of past RF in Australians and are consistent with those of researchers in the United States, Mexico, Russia, Chile and Israel.9-11,19,20 This adds to the growing evidence that D8/17 may be a universal marker of past RF in ethnically disparate populations. Negative findings by some US and north Indian researchers remain unexplained, but may reflect ethnic differences in the expression of B-cell antigens, selection of control patients, variation in antibody quality, or technical issues relating to the technique for selecting B cells.12-14,21
In previous reports, first-degree relatives have been shown to express D8/17 at intermediate levels, averaging 14.0% of B cells.9 Intermediate expression of D8/17 was also demonstrated in the relatives of patients with Sydenham’s chorea.20 These findings suggest that the B-cell marker is inherited, possibly along autosomal recessive lines. In our study, the absolute percentages of B cells positive for D8/17 in each category (patients with a past history of RF or RHD, first-degree relatives and healthy controls) reflect previous results and increase their reliability and generalisability.9
It is clear that D8/17 expression is not merely a marker of streptococcal infection, as it is not increased in poststreptococcal glomerulonephritis and uncomplicated streptococcal tonsillitis.9,22 However, post-streptococcal reactive arthritis is associated with elevated levels of D8/17, with D8/17 expression not conclusively differentiating between RF and poststreptococcal reactive arthritis in one small series.23
The functional significance of the D8/17 protein remains unknown. It does not match any known protein or HLA.20,24 The antibody to D8/17 has been shown to cross-react with cardiac muscle, skeletal muscle, smooth muscle cells of blood vessels and recombinant M6 streptococcal protein. Adsorption testing showed that B cells from a patient with RHD inhibited the binding of the D8/17 antibody to cardiac muscle. It was thus suggested that the D8/17 antigen may act as a streptococcal binding site on the B cells, and consequently become up-regulated after an infection, with B cells acting as antigen-presenting cells and influencing T-cell-specific cytotoxicity to heart and brain cells.22
A hypothesis is that D8/17, already expressed in excess in susceptible patients, is further augmented by the process that leads to RF. This suggests that D8/17 might be incorporated into the Jones criteria for the diagnosis of acute RF,17 either as an additional major manifestation, or as an obligatory feature in all diagnoses of acute RF. We are presently evaluating this in a larger, prospective study.
3 Comparison of mean D8/17 expression between categories of participants
Difference of the mean percentage of D8/17-positive B cells (95% CI) |
|||||||||||||||
- Zinta Harrington1
- Kumar Visvanathan2,3
- Narelle A Skinner3
- Nigel Curtis2
- Bart J Currie1,4
- Jonathan R Carapetis1,2,3
- 1 Menzies School of Health Research, Charles Darwin University, Darwin, NT.
- 2 Royal Childrens' Hospital, Melbourne, VIC.
- 3 Murdoch Childrens Research Institute, Melbourne, VIC.
- 4 Northern Territory Clinical School, Flinders University, Royal Darwin Hospital, Darwin, NT.
We thank the National Heart Foundation of Australia for funding that supported this work and the Cooperative Research Centre for Aboriginal and Tropical Health for providing scholarship assistance for Zinta Harrington. We also thank Rebecca Towers, Peter Fagan and Michael Harrington for assistance in the laboratory, Susan Jacups, and Ngalkanbuy Health for help in the field, and Professor John Zabriskie for providing D8/17 antibody.
None identified.
- 1. Cheadle W. Harbeian lectures on the various manifestations of the rheumatic state as exemplified in childhood and early life. Lancet 1889; 3426: 821-827.
- 2. Davies A, Lazarov E. Heredity, infection and chemoprophylaxis in rheumatic carditis: an epidemiologic study of a communal settlement. J Hygiene 1960; 58: 263-269.
- 3. Taranta A, Torosdag S, Metrakos J, et al. Rheumatic fever in monozygotic and dizygotic twins [abstract]. Circulation 1959; 20: 778.
- 4. DiSciascio G, Taranta A. Rheumatic fever in children. Am Heart J 1980; 99: 635-658.
- 5. Quinn R. Comprehensive review of morbidity and mortality trends for rheumatic fever, streptococcal disease and scarlet fever: the decline of rheumatic fever. Rev Infect Dis 1989; 11: 928-953.
- 6. Weidebach W, Goldberg A, Chiarella J, et al. HLA Class II antigens in rheumatic fever. Analysis of the DR locus by restriction fragment-length polymorphism and oligotyping. Hum Immunol 1994; 40: 253-258.
- 7. Guedez Y, Kotby A, El-Demellawy M, et al. HLA class II associations with rheumatic heart disease are more evident and consistent among clinically homogenous patients. Circulation 1999; 99: 2784-2790.
- 8. Visentainer J, Pereira F, Dalalio M, et al. Association of HLA-DR7 with rheumatic fever in the Brazilian population. J Rheumatol 2000; 27: 1518-1520.
- 9. Khanna A, Buskirk D, Williams R, et al. Presence of a non-HLA B cell antigen in rheumatic fever patients and their families as defined by a monoclonal antibody. J Clin Invest 1989; 83: 1710-1716.
- 10. Herdy G, Zabriskie J, Chapman F, et al. A rapid test for the detection of a B-cell marker (D8/17) in rheumatic fever patients. Brazil J Med Biol Res 1992; 25: 789-794.
- 11. Harel L, Zeharia A, Kodman Y, et al. Presence of the D8/17 B-cell marker in children with rheumatic fever in Israel. Clin Genet 2002; 61: 293-298.
- 12. Kaur S, Kumar D, Grover A, et al. Ethnic differences in expression of susceptibility marker(s) in rheumatic fever/rheumatic heart disease patients. Int J Cardiol 1998; 64: 9-14.
- 13. Taneja V, Mehra N, Reddy K, et al. HLA-DR/DQ antigens and reactivity to B cell alloantigen D8/17 in Indian patients with rheumatic heart disease. Circulation 1989; 80: 335-340.
- 14. Ganguly N, Anand I, Koicha M, et al. Frequency of D8/17 B lymphocyte alloantigen in north Indian patients with rheumatic heart disease. Immunol Cell Biol 1992; 70: 9-14.
- 15. Anonymous. Strategy for controlling rheumatic fever/rheumatic heart disease, with emphasis on primary prevention: memorandum from a joint WHO/ISFC meeting. Bull World Health Organ 1995; 73: 583-587.
- 16. Edmond K, Noonan S, Krause V, et al. The Top End rheumatic heart disease control program II. Rates of rheumatic heart disease and acute rheumatic fever. Northern Territory Dis Control Bull 2001; 8: 18-22.
- 17. Guidelines for the diagnosis of rheumatic fever. Jones Criteria, 1992 update. Special Writing Group of the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young of the American Heart Association. JAMA 1992; 268: 2069-2073.
- 18. Chapman F, Visvanathan K, Carreno-Manjarrez R, Zabriskie J. A flow cytometric assay for D8/17 B cell marker in patients with Tourette’s syndrome and obsessive compulsive disorder. J Immunol Methods 1998; 219: 181-186.
- 19. Gibofsky A, Khanna A, Suh E, Zabriskie J. The genetics of rheumatic fever: relationship to streptococcal infection and autoimmune disease. J Rheumatol 1991; 30 Supp 1: 1-5.
- 20. Feldman B, Zabriskie J, Silverman E, Laxer R. Diagnostic use of B-cell alloantigen D8/17 in rheumatic chorea. J Pediatr 1993; 123: 84-86.
- 21. Weisz J, McMahon W, Moore J, et al. D8/17 and CD19 expression on lymphocytes of patients with acute rheumatic fever and Tourette’s disorder. Clin Diagn Lab Immunol 2004; 11: 330-336.
- 22. Kemeny E, Husby G, Williams R, Zabriskie JB. Tissue distribution of antigen(s) defined by monoclonal antibody D8/17 reacting with B lymphocytes of patients with rheumatic heart disease. Clin Immunol Immunopathol 1994; 72: 35-43.
- 23. Zemel L, Hakonarson H, Diana D, Zabriskie J. Poststreptococcal reactive arthritis (PRSA): a clinical and immunogenetic analysis [abstract]. J Rheumatol 1992; 19 Suppl 33: 120.
- 24. Carreno-Manjarrez R, Viteri-Jackson A, Visvanathan K, Zabriskie J. Characterization of B-cell marker D8/17. In: Martin DR, Tagg JR, editors. Streptococci and streptococcal diseases: entering the new millennium. Proceedings of the XIV Lancefield International Symposium on Streptococci and Streptococcal Diseases, 1998. Auckland, New Zealand: Securacopy, 2000: 529-532.





Abstract
Objective: To test the B-cell antigen D8/17 as a marker of past rheumatic fever (RF) in a predominantly Aboriginal Australian population, and to evaluate technical modifications to allow its use in remote settings.
Design and setting: Cross-sectional survey in a remote Aboriginal community, a regional tertiary referral hospital and a tertiary paediatric centre in Melbourne.
Participants: 106 people, including three with acute RF, 38 with a history of past RF, 20 relatives of these people, and 45 healthy controls.
Main outcome measure: D8/17 expression in B cells.
Results: Blood was collected from each participant and the expression of D8/17 and CD19 in each sample was analysed by flow cytometry. The mean proportion of D8/17-positive B cells was 39.3% (SD, 11.8) in patients with previous RF, 22.5% (SD, 5.2) in first-degree relatives, 11.6% (SD, 7.2) in controls, and 83.7% (SD, 10.1) in patients with acute RF (analysis of variance test between means, P = 0.001). A cut-off of 22.1% of D8/17-positive B cells to indicate past RF yielded the highest percentage of correct results (95.4%). Delayed staining of whole blood (mean, 0.55 days; SD, 0.2) gave equivalent results to immediate staining, but the D8/17 assay on peripheral blood mononuclear cells was unreliable.
Conclusions: The B-cell antigen D8/17 accurately identifies Australians with a past history of RF, and the assay is feasible in remote settings with access to facilities capable of performing D8/17 staining within half a day of sample collection.