Infectious disease experts identify three prerequisites for pandemic influenza: the emergence of a new virus for which humans lack immunity, a virus capable of replication and causing disease in humans, and a type that is readily transmissible from human to human.1 The current outbreak in Asia of the H5N1 strain of avian influenza has met the first two preconditions. By late 2004, it was clear that avian influenza was established in the agricultural systems of Asia and was causing ongoing cases of human avian influenza infections (between December 2003 and 22 September 2005, 115 infections and 59 deaths were reported to the World Health Organization).2 As a result, the emergence of the third prerequisite is highly probable, and governments worldwide have moved to a raised level of pandemic preparedness.1
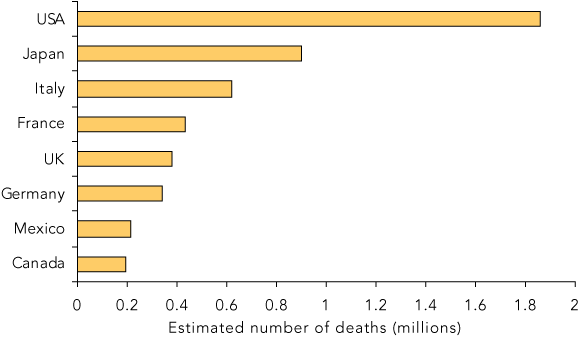
The global economic and human cost of pandemic influenza in the 21st century is likely to be very high. Depending on the infectivity of the virus, experts estimate that the worldwide toll could be between 7.4 and 150 million deaths (Box 1).3,4
The Australian Government estimates that a pandemic could result in up to 13 000 Australian deaths and 2.8 million infections.5 This number of infections is likely to overwhelm the health system and disrupt most economic activities. Given infection rates of up to 20% of the population and a global spread of disease occurring in waves over several months, the disruption to trade and industry could lead to a prolonged economic recession.
Vaccination is a key defence against influenza for individuals and populations. Vaccines, however, are unlikely to be available for at least the first 6 months of a pandemic. Targeted vaccine development requires isolation of the new pandemic strain, with further delays for safety testing and logistical constraints related to mass production, distribution and vaccination.1 Stockpiles of newer antiviral agents have therefore become a first-line strategy for governments, particularly for the first months of a pandemic until vaccines can be deployed en masse.
The neuraminidase inhibitor class of antivirals is active against most forms of influenza, is safer than older antivirals and, because of its long shelf-life, can be effectively stockpiled.1 Antivirals reduce virus shedding and thus infectivity of cases. Recent studies indicate targeted use can reduce transmission and potentially prevent a large outbreak or global pandemic.6 Many developed countries who can afford to establish stockpiles have ordered antivirals to cover 20%–40% of their populations.7 Owing to logistical benefits in relation to administration and stockpiling, countries have so far shown a preference for the tablet-form oseltamivir (proprietary name Tamiflu) rather than the inhaler preparation zanamivir (Relenza).
However, oseltamivir (and zanamivir, discussed below) is a relatively scarce resource, as it is under patent. In a licence agreement between Gilead Sciences and F Hoffmann-La Roche signed in 1996, Roche acquired an exclusive worldwide licence to all Gilead patent rights that cover an influenza neuraminidase inhibitor, its manufacture, formulation or use, including any intermediates used in manufacture. (A copy of the licence agreement is available at <www.gilead.com>.) The worldwide patent situation for oseltamivir is bound to be complex, as pharmaceutical companies will typically take out a cluster of patents around a given molecule. Ascertaining the patent status of a given molecule is a difficult matter and carries litigation risks if a wrong decision is made. We are not able to report on the patent situation around the world for oseltamivir, and it is probable that only Roche and Gilead know this with any certainty.
Patent laws grant exclusive rights over the manufacture and exploitation of products to the patent owner (for a minimum of 20 years in World Trade Organization [WTO] member countries, and up to 25 years in countries, like Australia, which allow patent term extensions). Under conditions of monopoly supply, the patent owner determines the price and volumes of production. Economic theory and empirical evidence indicate that the rational monopolist will set prices at the profit-maximising position, which is at higher prices and lower volumes than in a competitive market.8 Prices are set in relation to market conditions (“demand elasticity”) rather than production costs or the price at which societal benefit is optimised. The patented price of oseltamivir sets a potential limit on the quantities a country may decide to purchase, a limit that is likely to be significantly lower than if supply were subject to competition from generic pharmaceutical manufacturers.9,10
Importantly, decisions related to worldwide manufacturing capacity are also determined by the patent owner, whose commercial decisions as to supply may differ from the public health needs of countries. The commercial judgement of individual companies is likely to differ on issues of supply and distribution. Gilead, for example, has served a notice on Roche terminating their licence agreement, alleging that Roche has, among other things, failed to launch oseltamivir in markets around the world where it is registered.11 Similarly, the developer of zanamivir, the Australian biotechnology firm Biota, is currently suing GlaxoSmithKline (GSK), to whom it has sold the global licence for zanamivir, for failing to adequately exploit the product, including limited manufacturing and marketing of the therapy for pandemic preparedness (see <www.biota.com.au>).12 While Roche has expanded capacity in response to pandemic stockpiling, it is clear that this is not sufficient for many countries. Reports suggest there is currently a delay of 24 months for stockpile orders to be filled.13
The Federal Health Minister, Tony Abbott, has been very frank about the inadequacy of Australian stockpiles:
Certainly, we don’t have anything like enough antivirals to protect the entire population. At present, we have enough antivirals to protect one million essential service workers for about six weeks.14
He has also been very candid about supply constraints being a clear reason for the limited stockpiles:
[A]t the moment there are no additional antivirals anywhere in the world . . . If there were more antivirals to be had, by all means [we would expand stockpiles]. But on the best evidence we have, there aren’t.15
Providing such protection will be essential in order to ensure that workers such as police, doctors, nurses, water and electricity staff and airport employees turn up for work and maintain essential infrastructure. When supplies run out after 6 weeks or so, Australia will then be competing to obtain preferential treatment for a scarce resource from Roche.
In addition to its European plants, Roche recently announced it would build manufacturing capacity in the United States,16 which is not surprising given that its main markets are in the United States, Japan and Europe.17 Australia is a relatively inconsequential market for products sold by both Roche and GSK, and thus has compelling national security and public health reasons to look for alternatives to its current dependency on monopoly supply.
Similarly, although Australian taxpayers contributed significantly to the development of zanamivir, Australia does not have any preferential claim to its supply, as the licence for manufacture and distribution of zanamivir was sold by Biota to GSK.12 GSK has reportedly decided to re-establish manufacturing capacity for zanamivir in Australia, but these stocks will enter an international pool of supply to be distributed at the discretion of the UK-based multinational.18
However, antiviral capacity and supply is not fixed and need not be out of Australia’s control. In a 1992 study conducted by the United Nations Industrial Development Organization, Australia’s pharmaceutical industry was rated as both innovative and strong in manufacturing.19 Furthermore, it was Australian scientists at public institutions who pioneered the research that led to identification of the viral target and an Australian company, with the support of Australian Government grants, that developed the first neuraminidase inhibitor influenza antiviral to enter the market.20,21 While manufacture of the antiviral is reportedly complex, firms in Thailand and India have begun developing generic versions, and the Thai Government Pharmaceutical Organization announced it will begin mass production by October 2006 (though legal action by the patent owner is a concern).22 Given these developments, Australian scientists and generic pharmaceutical manufacturers should be able to reverse engineer and mass produce oseltamivir. However, this cannot occur until any possible patent barriers to generic production are addressed. Australia’s access to patented medicines is affected by its commitments as a member of the WTO. As a member, it is obliged to respect the patent provisions of the Agreement on Trade-Related Aspects of Intellectual Property Rights (the “TRIPS Agreement”).23 Another issue is the impact of the bilateral free trade agreement recently signed with the United States, which contains additional standards of intellectual property protection.24
If there were no blocking patents on oseltamivir registered in Australia, the Australian Government could begin exploring the option of generic production immediately. To secure certainty of supply, the government would have to find ways in which to make it commercially attractive for a generic company to invest in production. One way to assist a generic company to achieve economies of scale would be for the Australian Government to offer a company export assistance to those countries in Australia’s region that were in need of a stockpile and that had little prospect of meeting that need. There would be much strategic wisdom in Australia helping neighbouring countries in this way.
If, as is more probably the case, there turn out to be blocking patents on oseltamivir in Australia, the Australian Government could still pursue the generic option. It should first attempt to negotiate with the patent owner for a voluntary licence. If negotiations with patent owners fail to secure adequate supplies at affordable prices, the Australian Government could authorise the use of any relevant patents by a generic company. This is known as a “Crown use licence” (also referred to as a “government use licence” or a “compulsory licence”). This type of licence is expressly recognised under Article 31 of the TRIPS Agreement and is also allowable under the free trade agreement that Australia has with the United States. (This makes the lack of national discussion of this option particularly surprising). Patent owners would be entitled to adequate remuneration under such a licence, and thus would receive payments for doses it does not have the capacity to produce.
Even if Australia decides not to pursue the generic manufacturing option domestically, it should prepare itself for the issue of a compulsory licence. Firstly, it would provide the most effective means to force patent owners to compete on price and priority supply during purchasing negotiations. Secondly, if other countries such as Thailand or China decided to produce oseltamivir, they would be allowed, under Article 31 of the TRIPS Agreement, to export their surplus, provided that the bulk of their production (essentially 50% or more) was for domestic use. Before Australia could acquire any generic-product surplus, it would have to issue a compulsory licence.
Australia should also, as a matter of urgency, revisit its position under the WTO General Council decision of 30 August 2003.25 That decision sets up a system of export and import for pharmaceuticals under compulsory licence. Unfortunately, Australia followed the United States in declaring it would not use the system as an importer. The possibility of a pandemic and limited access to oseltamivir shows this position to be a high-risk gamble. Clearly, Australia needs to notify the WTO that it wishes to change its position. Australia could also use the system as an exporter, a decision that would not be constrained by the requirement that the bulk of production be for domestic supply. However, Australia would need to enact the necessary implementing legislation that would allow for export. Other countries such as Canada, the European Union, Norway and India have done so.
From the perspective of early control at source, it is in Australia’s interest to ensure optimal pandemic preparedness in countries that have had outbreaks of the H5N1 avian influenza strain and are likely sources of a pandemic. Most of the countries in our region that are potential sources of a pandemic strain are also the ones that can least afford to pursue a national stockpiling strategy at monopoly prices (Box 2), and many (such as Laos, Vietnam and Cambodia) do not have domestic manufacturing capacity. Australia’s donation of 50 000 courses of antiviral agents to Indonesia32 was essentially a symbolic gesture, given its population of 211 million.
Australia must promote compulsory licensing and generic production in the Asian region and support countries which are able to manufacture the drug to do so and to export it to countries that lack capacity (Box 3). The Foreign Minister could actively pursue this strategy at regional intergovernmental conferences such as the Asia–Pacific Economic Cooperation (APEC) bird-flu summit in late October 2005. Australia, as a key power in the region, has the opportunity (and responsibility) to create a climate of political confidence around the use of compulsory licensing as a tool of public health policy.
The Doha Declaration on the TRIPS Agreement and Public Health, signed by trade ministers of all WTO member countries on 14 November 2001, states in paragraph 4 that “the TRIPS Agreement does not and should not prevent members from taking measures to protect public health”.33 The same paragraph continues: “[T]he Agreement can and should be interpreted and implemented in a manner supportive of WTO members’ right to protect public health and, in particular, to promote access to medicines for all.”
Currently, antiviral agents are the only medical intervention available for influenza-affected patients. Privately, therefore, Australians are likely to demand universal access to this therapy and have a high level of willingness to pay. Clearly, if the manufacturers cannot meet demand at cost-effective prices, then there are health, economic and ethical arguments for a “government use” licence to be issued and for generic capacity to be developed and deployed rapidly in Australia.
To date, decision-makers have determined not to pursue this option or to even publicly discuss it. Furthermore, in view of limited supplies and likely overwhelming demand, a rationing system has been developed to determine a priority allocation list for these limited resources. For reasons of security, and also because of political pressures, the list of recipients has not been made publicly available. However, this process raises procedural and ethical questions in view of the fact that options for expanding access (eg, generic manufacture) are not being pursued by decision-makers, who are likely to be included in the list of essential public servants with access to national stockpiles. The policy of not pursuing generic production is further complicated by the fact that Australian taxpayers contributed to the early research that led to the discovery of the influenza target enzyme and subsequent development of antiviral therapies. (A 2003 study by Allens Consulting found that nearly 20% of the output of the biotechnology firm Biota, which developed the first neuraminidase inhibitor, could be attributed to Australian Government funding.)20,21
Ultimately, the questions of how to ensure adequate stockpiles, whether the generic antiviral option should be pursued, and whether governments have the resolve to use compulsory licences that are available under international and national laws to protect the health of nations is a contest of principles. It is a contest between patent monopolies, involving intellectual property rights, and the right to optimal access to essential medicines. Currently, decision-makers appear reluctant to challenge the interests of patent owners and the pharmaceutical industry. At a time of national pandemic alertness, they have, in a self-censoring fashion, failed to put the issue of compulsory licences and generic production on the table. We hope this article initiates an alternative debate.
2 Pandemic influenza and antiviral stockpiles: affordability and access
2.1 First movers in oseltamivir stockpiling race |
|
|
|
|
|||||||||||||||
Country |
Population in 2002 |
GDP per capita ($US)* |
Reported stockpile of 10-dose courses (millions) |
Estimated/reported expenditure on stockpile ($US million) |
Per capita expenditure on health in 2002 ($US)26 |
Estimated cost of oseltamivir course as a proportion of per capita health expenditure† |
|||||||||||||
288.4 |
36 123 |
20 |
1000 |
5274 |
0.95% |
||||||||||||||
Canada28 |
31.4 |
22 783 |
1.6 |
40 |
2222 |
1.13% |
|||||||||||||
United Kingdom29 |
58.9 |
26 376 |
14.6 |
253 |
2031 |
0.85% |
|||||||||||||
Australia30 |
19.6 |
20 969 |
3.9 |
88.92 |
1995 |
1.14% |
|||||||||||||
New Zealand31 |
3.9 |
15 033 |
0.8 |
nd |
1255 |
nd |
|||||||||||||
2.2 Selected countries affected by the H5N1 strain and affordability of oseltamivir stockpiles at patented prices |
|
|
|||||||||||||||||
|
Population in 2002 |
GDP per capita ($US)* |
Stockpile required to cover 20% of population (millions of courses) |
Estimated cost of stockpile at patented prices ($US million) |
Per capita expenditure on health in 2002 ($US)26 |
Estimated cost of oseltamivir course as proportion of per capita health expenditure‡ |
|||||||||||||
China |
1281.0 |
966 |
256.2 |
4439.5 |
63 |
28% |
|||||||||||||
Cambodia |
12.5 |
294 |
2.5 |
43.3 |
32 |
54% |
|||||||||||||
Indonesia |
211.7 |
817 |
42.3 |
733.8 |
26 |
67% |
|||||||||||||
Lao People's Democratic Republic |
5.5 |
304 |
1.1 |
19.2 |
10 |
173% |
|||||||||||||
Vietnam |
80.5 |
436 |
16.1 |
279.1 |
23 |
75% |
|||||||||||||
GDP = gross domestic product. nd = no data. * Source: World Development Indicators database, World Bank, July 2003. †Estimate based on average prices calculated from reported volume and cost of stockpiles. ‡Estimate based on lowest reported price in Table 2.1 of $US17.3 for the United Kingdom. |
|||||||||||||||||||
3 Antivirals and preparedness for a regional pandemic of avian influenza
The Australian Government should:
Announce that it will investigate the issue of a compulsory licence and local generic manufacture of influenza antivirals
Explore the possibility of exporting any generic surplus to neighbouring countries at high risk of a pandemic
Express public and strong support for countries in the region taking the compulsory licensing route
Raise the issue of a coordinated approach to generic manufacture in the region
- Buddhima Lokuge1
- Peter Drahos2
- Warwick Neville3
- 1 Medical School, Australian National University, Canberra, ACT.
- 2 Centre for Governance of Knowledge and Development, Regulatory Institutions Network, Australian National University, Canberra, ACT.
Buddhima Lokuge has received financial support from the Australian Research Council grant titled “The impact of international trade agreements on the regulation and provision of medicines in Australia”. The Australian Research Council had no input into the writing of this article.
None identified.
- 1. World Health Organization. WHO consultation on priority public health interventions before and during an influenza pandemic. Geneva: WHO, 2004.
- 2. World Health Organization. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. Available at: http://www.who.int/csr/disease/avian_influenza (accessed Oct 2005).
- 3. Stohr K, Esveld M. Will vaccines be available for the next influenza pandemic? Science 2004; 306: 2195-2196.
- 4. Department of Public Information, News and Media Division, United Nations. Press conference by UN System Senior Coordinator for Avian and Human Influenza [press briefing]. New York, 29 September 2005. Available at: http://www.un.org/News/briefings/docs/2005/050929_Nabarro.doc.htm (accessed Oct 2005).
- 5. Abbott T, MP, Federal Minister for Health. The odds shorten on Australia facing a deadly test of its national psyche. Sydney Morning Herald 2005 Sep 16. Available at: http://www.smh.com.au/news/opinion/the-odds-shorten-on-australia-facing-a-deadly-test-of-its-nationalpsyche/2005/09/15/1126750072714.html (accessed Oct 2005).
- 6. Ferguson NM, Cummings DA, Cauchemez S, et al. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature 2005; 437: 209-214.
- 7. F Hoffman-La Roche Ltd. Roche donates 3 million treatments of antiviral Tamiflu to the WHO for use in an influenza pandemic [media release]. Basel, 24 August 2005. Available at: http://www.roche.com/med-cor-2005-08-24 (accessed Oct 2005).
- 8. Baumol WJ, Blinder AS. Microeconomics: principles and policy. 8th ed. New York: Harcourt, 2000.
- 9. Stohr K. Preventing and treating influenza. BMJ 2003; 326: 1223-1224.
- 10. US Federal Trade Commission. Generic drug entry before patent expiration: an FTC study. Washington, DC: FTC, 2002.
- 11. Gilead Sciences, Inc. Gilead delivers termination notice to Roche for Tamiflu development and licensing agreement [media release]. Foster City, Calif, 23 Jun 2005. Available at: http://www.gilead.com/wt/sec/pr_723430 (accessed Oct 2005).
- 12. Biota sues GSK — a sensible tactic to recover up to $150 million in lost royalties. Bioshares 2005; 69: 1-3. Available at: http://esvc001029.wic014u.server-web.com/presentations/pdf/Bioshares-BTA-No69-May04.pdf (accessed Oct 2005).
- 13. World Health Organization. Donation of three million treatments of oseltamivir to WHO will help early response to an emerging influenza pandemic [media release]. Geneva, 24 August 2005. Available at: http://www.who.int/mediacentre/news/releases/2005/pr36/en/index.html (accessed Oct 2005).
- 14. Limb J. Government prepares but says bird flu pandemic isn’t probable [transcript of radio program]. “PM”, ABC Radio National Australia, 28 Sep 2005. Available at: http://www.abc.net.au/pm/content/2005/s1470530.htm. 2005 (accessed Oct 2005).
- 15. Jones T. Australia prepares for potential bird flu pandemic [transcript of television program]. “Lateline”, Australian Broadcasting Corporation, 17 Sep 2005. Available at: http://www.abc.net.au/lateline/content/2005/s1459628.htm. (accessed Oct 2005).
- 16. US Department of Health and Human Services. HHS buys vaccine and antivirals in preparation for a potential influenza pandemic [media release]. Washington, DC, 15 Sep 2005. Available at: http://www.hhs.gov/news/press/2005pres/20050915.html (accessed Oct 2005).
- 17. F Hoffman-La Roche Ltd. Annual report 2004. Basel: Roche, 2004.
- 18. Gluyas R. Flu drug will be made in Australia. The Australian 2005 Oct 18; The Nation. http://www.theaustralian.news.com.au/common/story_page/0,5744,16952572%255E2702,00.html (accessed Oct 2005, no longer available).
- 19. Ballance R, Pogany J, Forstner H. The world’s pharmaceutical industries: an international perspective on innovation, competition and policy. Prepared for the United Nations Industrial Development Organization (UNIDO). Cheltenham, UK: Edward Elgar Publishing, 1992.
- 20. Laver WG, Bischofberger N, Webster RG. The origin and control of pandemic influenza. Perspect Biol Med 2000; 43: 173-192.
- 21. Allen Consulting Group. The economic impact of the commercialisation of publicly funded R & D in Australia. Report prepared on behalf of the Australian Institute for Commercialisation. Melbourne: Allen Consulting Group, 2003 Available at: http://www.allenconsult.com.au/resources/AIC_ACG_Report.pdf (accessed Oct 2005).
- 22. Wipatayotin A, Jongcharoen P. GPO to make Thai version of Tamiflu. Bangkok Post 2005 Oct 18. Available at: http://lists.essential.org/pipermail/ip-health/2005-October/008401.html (accessed Oct 2005).
- 23. Barton JH. TRIPS and the global pharmaceutical market. Health Aff (Millwood) 2004; 23: 146-154.
- 24. Drahos P, Henry D. The free trade agreement between Australia and the United States. BMJ 2004; 328: 1271-1272.
- 25. World Trade Organization. Implementation of paragraph 6 of the Doha Declaration on the TRIPS Agreement and Public Health. Decision of the General Council of 30 August 2003. Available at: http://www.wto.org/english/tratop_e/trips_e/implem_para6_e.htm (accessed Oct 2005).
- 26. World Health Organization. The World Health Report 2005. Annex table 6. Selected national health accounts indicators: measured levels of per capita expenditure on health, 1998–2002. Available at: http://www.who.int/whr/2005/annex/annexe6_en.pdf (accessed Oct 2005).
- 27. Armitage T. US talks on Roche flu drug deal continue. Reuters, 19 Sep 2005. Available at: http://www.boston.com/news/world/europe/articles/2005/09/19/us_talks_on_roche_flu_drug_deal_continue (accessed Oct 2005).
- 28. Public Health Agency of Canada. Minister Dosanjh announces federal contribution towards creation of a national antiviral stockpile [media release]. Vancouver, 4 Feb 2005. Available at: http://www.phac-aspc.gc.ca/media/nr-rp/2005/2005_3_e.html (accessed Oct 2005).
- 29. UK Department of Health. Improving preparedness for possible flu pandemic. Purchase of antiviral drugs and publication of plan [media release]. 1 Mar 2005. No. 2005/0083. Available at: http://www.dh.gov.uk/PublicationsAndStatistics/PressReleases/PressReleasesNotices/fs/en?CONTENT_ID=4104448&chk=J4vpsy (accessed Oct 2005).
- 30. Australian Government Department of Health and Ageing. Health Fact Sheet 1 — Addressing emerging or potential health risks. Budget 2004–2005, 11 May 2004. Available at: http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-budget2004-hbudget-hfact1.htm (accessed Oct 2005).
- 31. Roche negotiating Tamiflu deal ahead of possible bird flu pandemic. Reuters, 2 Mar 2005. Available at: http://www.msnbcenespanol.com/id/7067756/ (accessed Oct 2005).
- 32. Downer A, MP, Federal Minister for Foreign Affairs. Australian experts to assist Indonesia combat avian influenza [media release]. Canberra, 4 October 2005. No. FA123. Available at: http://www.foreignminister.gov.au (accessed Oct 2005).
- 33. World Trade Organization. Declaration on the TRIPS Agreement and Public Health. Ministerial Conference, Fourth Session, Doha, 9–14 Nov 2001. WT/MIN(01)/DEC/2, 20 November 2001. Available at: http://www.sice.oas.org/trade/WTODoha/mindecl_trips_e.asp (accessed Oct 2005).





Abstract
In view of the possibility of a human pandemic of avian influenza, a first-line strategy for many countries is stockpiling of antiviral neuraminidase inhibitors (oseltamivir [Tamiflu] and zanamivir [Relenza]), which can reduce mortality, morbidity and influenza transmission.
However, global supply of the antivirals is controlled by the European-based patent owners, Roche and GlaxoSmithKline. This prevents competition in the manufacturing and distribution of antivirals and has reduced global supply capacity and affordability.
The Australian Government has acknowledged that, in the event of a pandemic, its own stockpile of antivirals will be limited and reserved for those on a confidential rationing list. Pharmacies are running out of stocks, limiting opportunities for individuals to secure supplies privately.
Compulsory licensing provisions, permitted under domestic patent law, would allow Australian generic manufacturers to start producing antivirals locally or import them from generic producers at affordable prices.
Australia also has an opportunity and a responsibility to promote compulsory licensing and generic antiviral production in the Asian region, to ensure our neighbours can establish pandemic stockpiles in a timely and affordable manner.