Coronary artery disease is a major health and economic burden in developed countries. Some survivors of an acute coronary event are offered rehab-ilitation to accelerate recovery and reduce the high risk of recurrence.1 Exercise-based rehabilitation programs may improve quality of life in selected patients,2 reduce health care costs of associated treatment,3-5 modify coronary risk factors,6 and reduce mortality.2,7 However, evidence for the cost-effectiveness of cardiac rehabilitation is based on a few studies with limited cost analyses.8 The only published randomised trial was limited to patients with psychological impairment after myocardial infarction and reported modest improvements in quality of life and fewer visits to community rehabilitation programs.4
We report the outcomes of a randomised controlled trial examining the cost-effectiveness of exercise-based rehabilitation of patients after hospital admission for an acute coronary syndrome event.
Participants were allocated at random to hospital-based cardiac rehabilitation or conventional care and followed up for 12 months. Central randomisation of participants was performed at the National Health and Medical Research Council Clinical Trials Centre using dynamic balancing,9 hierarch-ically stratified according to the recruiting hospital, age ≤ 65 or > 65 years, sex, transmural anterior myocardial infarction or not, and percutaneous transluminal coronary angioplasty (PTCA) during index admission or not.
Aerobic circuit training interspaced with resistance training was the focus of the rehab-ilitation programs at both participating institutions. Non-exercise sessions addressed symptom management, pharmacological treatment, healthy eating, psychosocial counselling and stress management delivered by a clinical nurse consultant, physiotherapist, clinical psychologist, dietitian, social worker or pharmacist. Exercise testing before and after the program was not routinely performed, nor was electrocardiographic monitoring during supervised exercise. The rehabilitation programs at both institutions were conducted in accordance with published recommendations for postdischarge cardiac rehabilitation.10
All cardiovascular hospitalisations during the follow-up period were included and assigned a cost, based on the diagnosis-related group (DRG) category reference and length of stay.11 Cardiovascular medications, visits to health professionals and investigations were valued according to the Schedule of Pharmaceutical Benefits,12 the Australian Medicare Benefits Schedule and the Australian Pharmaceutical Benefits Scheme’s manual of resource items and their associated costs,13 respectively. Patients and their general practitioners provided health resource utilisation data by completing comprehensive questionnaires at 6 and 12 months. Hand searching of medical records was used to determine and verify all outpatient medical investigations, treatments and risk factor measurement after hospital admissions. Where discrepancies arose between medical records and patient reports, the former was taken as the more complete document. Information not available from the patient was taken from the treating doctor’s medical records.
Costs are calculated in 1998 Australian dollars.14,15 Costs of providing cardiac rehab-ilitation were taken from a health system perspective and calculated over 1 year; they included staffing, consumables, overheads and capital expenses.
Quality-of-life measures were estimated at baseline, and at 6 and 12 months, using the standard Medical Outcomes Study Short Form-36 (SF-36) questionnaire16 and the disease-specific Utility-Based Quality of life–Heart questionnaire (UBQ-H).17 The UBQ-H was developed specifically for use in coron-ary artery disease and has been validated in this clinical situation.17,18 Components of the UBQ-H include physical ability, psychological distress, and social/usual activities, plus three summary measures of quality of life, including a time trade-off item, a rating scale and an ordinal health assessment item. The UBQ-H was used to assign the patient’s utility (preference) for their current health state and to calculate QALYs by treatment arm, integrating UBQ-H utility scores (0–1 scale) and survival by treatment arm using the Quality Adjusted Survival Analysis (QASA) method.19 Utility is defined by the Department of Health and Ageing20 as a numerical value assigned by an individual to a preference for, or a desirability of, a specific level of health status or a specific health outcome (on a scale of 1 = full health and 0 = death).
Incremental cost per QALY saved was calculated as an incremental (rehabilitation v conventional care) cost per patient divided by the incremental QALYs per patient. Base-case analysis estimated life years accrued to 1 year from the treatment effect on mortality in a published meta-analysis of randomised trials of rehabilitation with exercise.7 Alternative cases of no treatment effect on survival and 3 years of treatment effect7 were modelled in sensitivity analyses.
The SF-36 guides suggest that a difference of 10 points between groups per health domain indicates a clinically worthwhile difference.21 Sample size calculations revealed that about 50 patients per treatment arm would allow 80% power for detecting such a difference (assuming an 18-point standard deviation) in each of the SF-36 health domains with P = 0.05. A total of 100 patients would also offer greater than 80% power to detect a clinically worthwhile 0.1 ± 0.2 SD difference in utility scores on the UBQ-H questionnaire.17,18 Shapiro–Wilk tests were used to test for normally distributed data. Unpaired t tests, χ2 tests and Mann–Whitney U tests for non-normal data were used for comparisons between groups at baseline. For differences between groups over time, longitudinal multiple regression analysis and repeated-measures ordinal regression analysis were undertaken using Statview 5 (SAS Institute Inc, Cary, NC, USA) and Accord (Boffin Software, NSW), respectively. Statistical significance was inferred if 2-tailed tests estimated P < 0.05. All results are presented unadjusted for multiple comparisons and all analyses were done on an intention-to-treat basis. Ninety-five per cent CIs for the difference in QALYs saved between treatment arms were calculated with bootstrapping.22
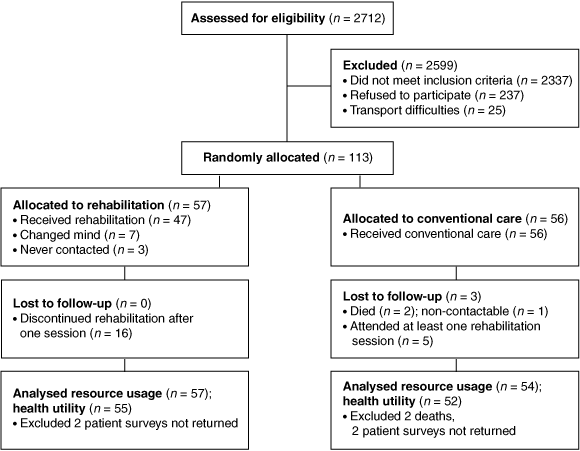
A total of 375 patients met the eligibility criteria for the study and 113 (30%) of these were randomised between rehabilitation (n = 57) and conventional care (n = 56). The randomised groups were well balanced for baseline characteristics (Box 1). For various reasons, the remainder elected not to participate: no interest (105), a perception that rehabilitation was unnecessary or inconvenient (90), immediate return to work (42), and transport difficulties (25). Another 2337 (86%) screened subjects were considered ineligible: 1347 resided outside the area health service, 397 were aged > 75 years, 285 had a comorbidity, 255 had insufficient English literacy, 25 had a definite indication for rehabilitation (and were subsequently referred), 23 were not interviewed, and five were in deteriorating health (Box 2).
Baseline estimates (conventional v rehabilitation group) of health-related utility elicited using the UBQ-H were not significantly different (0.9599 and 0.9593, respectively; P = 0.47 for difference between trial arms). Utility scores increased progressively relative to this base level in both treatment arms over 12 months. At 6 months, the mean incremental improvements in health utility were 0.012 (95% CI, 0.002 to 0.023) for conventional care and 0.016 (95% CI, 0.006 to 0.026) for rehabilitation care, respectively (P = 0.78 for difference between trial arms). At 12 months, there was a non-significant improvement from baseline of 0.010 (95% CI, − 0.001 to 0.022) in conventional care and a significant improvement of 0.026 (95% CI, 0.013 to 0.039) in rehabilitation care. While the estim-ated improvement in utility was higher in rehabilitation patients at 12 months, the difference between improvements in conventional and rehabilitation care was not significant (P = 0.38).
Similar results were seen for SF-36 domain scores: domain scores improved from baseline at 6 and 12 months, with non-significant advantages for rehabilitation across domains except for “physical function” where a significant advantage was seen at 12 months (P = 0.04, Box 3).
Using utility estimates at 6 and 12 months, and published survival effects at 1 year of 21.2%7 applied to the base risk mortality of 5.7% in an Australian population after AMI,23 the estimated gain in QALYs was 9.289 per 1000 up to 12 months.
Resource use costs for both groups are shown in Box 4. The incremental costs per patient of rehabilitation services relative to conventional care were estimated at $631 for rehabilitation services, $51 for test and investigation costs, $20 for patient expenses and $19 for hospital costs. These additional costs of rehabilitation were partially offset by lower costs for medication ($117), ambulance ($143), and other consultations ($66). Overall, resource utilisation at 12 months was non-significantly higher for the rehabilitation group by an amount of $395 per patient (P = 0.74).
During follow-up, 19 patients from each group required readmission on a total of 65 occasions for cardiovascular indications. This included 19 versus 10 admissions for angina for the conventional and rehabilitation groups, respectively; five versus two admissions for other chest pain; four versus three admissions for PTCA; zero versus four admissions for coronary graft surgery; one admission each for AMI; three versus two admissions for arrhythmia; one versus zero admissions for automated implantable cardiac defibrillator; and four versus six admissions for “other cardiovascular causes”. The total number of readmissions was non-significantly higher for the conventional group (36 v 29; P = 0.56). Base-case analyses excluded the cost of an admission for defibrillator implantation in the conventional group ($47 305). Inclusion of the cost of this defibrillator would have resulted in an overall incremental cost saving of $450 per patient for the rehabilitation group.
Box 5 summarises the results from the one-way sensitivity analyses performed to determine resilience to variations around base-case estimates. The incremental cost per QALY saved was most sensitive to variations in the utility scores. Ninety-five percent CIs for incremental utility were estimated by bootstrapping the sampled distribution of incremental utility with 10 000 replicates re-sampled with replacement from treatment and control populations. The 95% CI for incremental QALYs saved ranged from 0.020 down to − 0.001. At the upper 95% confidence limit for QALYs saved, the expected cost per QALY saved was $19 690, but rehabilitation treatment was dominated at the upper boundary and was therefore not cost-effective. Varying the relative risk reduction across the range of its 95% CI (− 2.8% to 39% relative risk reduction in mortality)7 resulted in an estimated cost-effectiveness ratio from $77 250 down to $31 630 per QALY saved. Adjusting the base-rate mortality to half and twice its published rate resulted in an estimated cost per QALY saved of $30 970 and $52 300, respectively.
An alternative assumption of no treatment effect on baseline survival increased the cost per QALY saved to $70 580 (attributable to effects on quality of life alone), while estim-ating QALYs saved up to 3 years, by applying the 36-month relative risk reduction to the baseline mortality risk for a 3-year analysis, reduced the cost per QALY saved to $27 030.
This Australian trial is one of the first randomised evaluations worldwide of the cost-effectiveness of cardiac rehabilitation in a broad cross-section of typical patients with acute coronary syndromes. Estimates of quality of life derived from our study show a non-significant greater gain from rehabilitation up to 1 year. Rehabilitation costs of $631 per patient were offset by a reduction in follow-up costs of $236, resulting in a net incremental cost per patient of $395.
The instruments we used for assessing quality of life were sensitive to patient change, as evidenced by the statistical significance of improvement over time in the cohort as a whole. Additional to the improvements seen in the conventional care group, early exercise-based rehabilitation was associated with small but consistently favourable changes in utility and health states of SF-36 domains, similar to previous studies using other quality-of-life instruments.24-27 Improvement in self-reported physical function suggested higher levels of daily physical activity in response to rehabilitation during follow-up. The size of the difference equates to 2 of 10 tasks of daily living performed with little or no limitation, compared with substantial previous limitation, and would therefore be clinically important. Similar subjective improvements in physical function after rehabilitation have been found24,26,28-32 and confirmed by objective improvements in functional capacity.33-36 These results support the notion that early exercise-based rehabilitation counters functional cardiac impairment.
The major limitations of our study included the relatively small sample size, the incomplete compliance with rehabilitation, and the use of pre-thrombolysis estimates for the mortality benefits of exercise-based rehabilitation.
The small sample size is likely to have limited the ability to detect reliably smaller, yet possibly still clinically important, changes that may exist in quality of life. Our study, while excluding 10-point advantages from rehabilitation in SF-36 health domains21 at 6 and 12 months with 5% type I and 20% type II error, does suggest the potential for smaller advantages which could be investigated with larger trials.
Both the level of non-compliance with allocation to rehabilitation and some cross-over from the conventional arm could be expected to have diluted the measured effects of rehabilitation.
Treatment effects on survival observed before the routine use of thrombolysis may no longer accurately reflect the potential survival advantage following on from rehab-ilitation, as the case-fatality rate of myocardial infarction has fallen with the use of thrombolytics37 and, more recently, primary angioplasty.38 However, treatment effects of rehabilitation on mortality have been conservatively modelled. Recent Australian cohort data for the post-thrombolytic era by Sundararajan and colleagues suggest a 35% improvement in 5-year survival was associated with rehabilitation attendance.23
DRG costs were used for re-admission to hospital during follow-up and represent average costs of treatment for those patients, which may not fully reflect the actual inpatient care costs.11
The findings of our study strengthen the case for rehabilitation services to be made available and routinely offered to all survivors of acute coronary syndromes. The advantages in QOL were mostly non-significant, but the cost of delivering rehabilitation was low. The estimated incremental cost per QALY saved of $42 535, derived under conservative assumptions, is therefore consistent with those accepted by decision-making authorities such as the Pharmaceutical Benefits Advisory Committee.39
These findings are particularly important given recent estimates suggesting that less than 30% of Australian patients have access to rehabilitation programs after an acute coronary syndrome.40 Alternative strategies for the delivery of cardiac rehabilitation, with more explicit cooperation and agreed pro-cesses between the rehabilitation team and doctors providing conventional care, may achieve better health outcomes and further enhance its cost-effectiveness.
1 Baseline characteristics of the patients in the conventional care and rehabilitation groups. Data are number of patients or mean (SD)
Characteristics |
Conventional (n = 56) |
Rehabilitation (n = 57) |
|||||||||||||
Physical characteristics |
|
|
|||||||||||||
Sex (M : F) |
42 : 14 |
41 : 16 |
|||||||||||||
Age (years) |
60.8 (8.7) |
61.9 (9.4) |
|||||||||||||
Body mass index (kg·m-2) |
26.7 (5) |
26.1 (4) |
|||||||||||||
Clinical details on index admission |
|
|
|||||||||||||
Index diagnosis — AMI : unstable angina |
27: 29 |
21: 36 |
|||||||||||||
Thrombolytic therapy |
14 |
8 |
|||||||||||||
PTCA/CAGS |
26 |
34 |
|||||||||||||
Prior AMI, PTCA, CAGS |
28 |
21 |
|||||||||||||
Prior cardiac rehabilitation |
3 |
3 |
|||||||||||||
Coronary risk factors* |
|
|
|||||||||||||
Family history of coronary artery disease |
13 |
13 |
|||||||||||||
Hypercholesterolaemia |
26 |
25 |
|||||||||||||
Hypertension |
29 |
25 |
|||||||||||||
Current smoker |
20 |
17 |
|||||||||||||
Diabetes |
9 |
6 |
|||||||||||||
Obesity |
12 |
4 |
|||||||||||||
Medication at randomisation |
|
|
|||||||||||||
Aspirin |
50 |
53 |
|||||||||||||
Antiarrhythmic agent |
5 |
3 |
|||||||||||||
β-blocker |
36 |
35 |
|||||||||||||
Angiotensin-converting enzyme inhibitor |
17 |
15 |
|||||||||||||
Calcium antagonist |
22 |
15 |
|||||||||||||
Long acting nitrate |
34 |
32 |
|||||||||||||
Diuretic |
6 |
6 |
|||||||||||||
Insulin |
4 |
2 |
|||||||||||||
Hypolipidaemic agent |
12 |
16 |
|||||||||||||
Oral hypoglycaemic agent |
3 |
3 |
|||||||||||||
All characteristics were well-balanced between allocated groups, except for obesity (P = 0.03, unadjusted for multiple comparisons). P values are for differences between conventional and rehabilitation groups using unpaired t test, χ2 or Mann–Whitney U test. AMI = acute myocardial infarction. PTCA = percutaneous transluminal coronary angioplasty. CAGS = coronary artery graft surgery. * Family history of coronary artery disease: first degree relative aged < 60 years with an acute coronary event; hypercholesterolaemia: total cholesterol level, ≥ 4.5 mmol/L; hypertension: blood pressure, ≥ 140/90 mmHg; diabetes: fasting plasma glucose level, ≥ 7.8 mmol/L; obesity: body mass index, > 30 kg·m-2). |
|||||||||||||||
2 Progress of participants in the trial of rehabilitation v conventional care after an acute coronary syndrome event
3 Mean (95% CIs) baseline SF-36 scores* and change in SF-36 scores at 6 and 12 months for conventional and rehabilitation groups
Quality of life measure (SF-36) |
Baseline SF-36 score (n = 113) |
Change at 6 months |
Change at 12 months |
||||||||||||
Conventional (n = 53) |
Rehabilitation (n = 56) |
Conventional (n = 51) |
Rehabilitation (n = 55) |
||||||||||||
Physical function |
61.9 (56 to 66) |
7.1 (1 to 13) |
15.9 (− 8 to 23) |
6.8 (− 1 to 14) |
17.6‡ (10 to 25) |
||||||||||
Role physical† |
0.0 (0 to 75) |
75.0 (0 to 100) |
75.0 (0 to 100) |
75.0 (25 to 100) |
100.0 (0 to 100) |
||||||||||
Bodily pain |
48.9 (44 to 54) |
19.2 (11 to 27) |
26.6 (18 to 35) |
20.9 (12 to 30) |
30.2 (23 to 37) |
||||||||||
General health |
59.6 (55 to 63) |
− 0.6 (− 5 to 4) |
0.1 (− 6 to 6) |
2.2 (− 2 to 7) |
2.7 (-3 to 9) |
||||||||||
Vitality |
50.9 (46 to 55) |
3.7 (− 2 to 9) |
7.1 (1 to 13) |
6.9 (1 to 12) |
11.9 (6 to 18) |
||||||||||
Social function |
59.2 (55 to 66) |
14.1 (7 to 21) |
19.6 (10 to 29) |
16.4 (9 to 23) |
23.6 (14 to 33) |
||||||||||
Role emotional† |
66.7 (0 to 100) |
33.3 (33 to 100) |
33.3 (0 to 100) |
33.3 (33 to 100) |
33.3 (33 to 100) |
||||||||||
Mental health |
70.8 (67 to 75) |
1.4 (− 3 to 5) |
0.5 (− 4 to 5) |
3.9 (0 to 8) |
3.6 (-1 to 9) |
||||||||||
* Domains measured out of 100. SF-36 = Medical Outcomes Study Short Form-36 questionnaire. |
|||||||||||||||
4 Cost of health resource use during the 12-month follow-up (1998 nearest Australian dollars)
Health resource |
Mean cost per patient conventional |
Mean cost per patient rehabilitation |
Incremental cost (rehabilitation minus conventional) |
||||||||||||
DRG costs* |
$2257 |
$2276 |
$19 |
||||||||||||
Pharmaceutical costs† |
$783 |
$666 |
− $117 |
||||||||||||
Test costs‡ |
$526 |
$577 |
$51 |
||||||||||||
Consultation costs§ |
$472 |
$406 |
− $66 |
||||||||||||
Rehabilitation costs¶ |
$63 |
$694 |
$631 |
||||||||||||
Patient expenses** |
$109 |
$129 |
$20 |
||||||||||||
Ambulance costs |
$332 |
$189 |
− $143 |
||||||||||||
Mean total cost per patient†† |
$4541 |
$4937 |
$395 |
||||||||||||
* Excludes defibrillator costs for a patient in the conventional treatment arm. †Includes angiotensin converting enzyme (ACE) inhibitors, β-blockers, diuretics, calcium antagonists, lipid-lowering drugs, nitrates, amiodarone, aspirin, digoxin, disopyramide, prazosin, warfarin, insulin and hypoglycaemic agents. ‡Includes echo Doppler test, exercise test, nuclear scan, electrocardiogram, chest x-ray, and blood test. §Includes visits to the emergency department, local doctor, heart specialist, or dietitian. ¶Includes the cost of rehabilitation sessions. ** Includes direct expenses borne by patient (see text). ††Cost estimates to the nearest Australian dollar. DRG = diagnosis-related group. |
|||||||||||||||
5 One-way sensitivity analyses of base case incremental cost effectiveness ($ per quality-adjusted life year [QALY] saved)
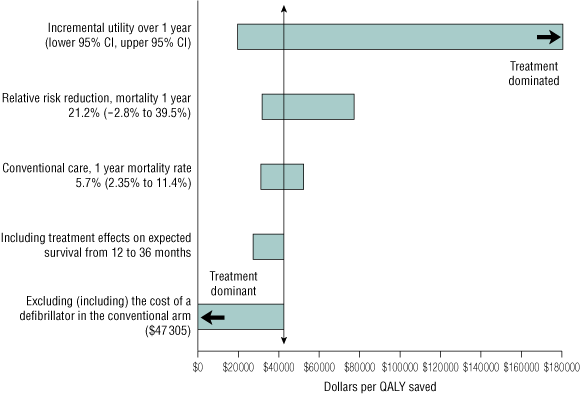
The base-case estimate is $42 535 per QALY saved at 1 year (includes expected life years to 1 year from evidence-based survival effect). The sensitivity range is shown in parentheses.
Received 30 March 2005, accepted 29 August 2005
- Tom G Briffa1
- Simon D Eckermann2
- Alison D Griffiths3
- Anthony C Keech4
- Phillip J Harris5
- M Rose Heath6
- Saul B Freedman7
- Lana T Donaldson8
- N Kathryn Briffa9
- 1 NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW.
- 2 Cardiology Department, Royal Prince Alfred Hospital, Camperdown, NSW.
- 3 Department of Cardiology, Concord Repatriation General Hospital, Concord, NSW.
- 4 School of Physiotherapy, Curtin University, Perth, WA.
The authors thank Rhana Pike (NHMRC Clinical Trials Centre) for editorial assistance with the manuscript, and Philip Ades for helpful comments and advice.
Tom Briffa was awarded the following scholarships to conduct the study: the Faculty of Medicine at the University of Sydney (2 years), the Cardiac Society of Australia and New Zealand (1 year), and the National Heart Foundation of Australia (1 year). Anthony Keech was supported through a Fellowship Grant from the NHMRC. Support for the work was also provided by the Department of Cardiology, Royal Prince Alfred Hospital and the NHMRC Unit Grant awarded to the Clinical Trials Centre.
None identified.
- 1. Ades P. Cardiac rehabilitation and secondary prevention of coronary heart disease. N Engl J Med 2001; 345: 892-902.
- 2. Oldridge N, Guyatt G, Fischer M, Rimm A. Cardiac rehabilitation after myocardial infarction: combined experience of randomised trials. JAMA 1988; 260: 945-950.
- 3. Levin L, Perk J, Hedback B. Cardiac rehabilitation — a cost analysis. J Intern Med 1991; 230: 427-434.
- 4. Oldridge N, Furlong W, Feeny D, et al. Economic evaluation of cardiac rehabilitation soon after acute myocardial infarction. Am J Cardiol 1993; 72: 154-161.
- 5. Ades P, Huang D, Weaver S. Cardiac rehabilitation participation predicts lower rehospitalization costs. Am Heart J 1992; 123: 916-921.
- 6. Wenger N, Froelicher E, Smith L, et al. Cardiac rehabilitation. Clinical practice guideline no. 17. Rockville, Md: Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research and the National Heart, Lung, and Blood Institute, 1995.
- 7. O’Connor G, Buring J, Yusuf S, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation 1989; 80: 234-244.
- 8. McAlister F, Lawson F, Teo K, Armstrong P. Randomised trials of secondary prevention programmes in coronary heart disease: systematic review. BMJ 2001; 323: 957-962.
- 9. Signorini D, Leung O, Simes R, et al. Dynamic balanced randomisation for clinical trials. Stat Med 1993; 12: 2343-2350.
- 10. National Heart Foundation of Australia. Minimal standards for outpatient (phase 2) cardiac rehabilitation and cardiac rehabilitation policy statements. Canberra: NHFA, 1994.
- 11. Casemix standards for NSW 1996–1997. Sydney: NSW Health, 1998.
- 12. Commonwealth Department of Human Services and Health. Schedule of pharmaceutical benefits for approved pharmacists and medical practitioners. Canberra: AGPS, 1998.
- 13. Pharmaceutical Benefits Scheme. Manual of resource items and their associated costs for use in submissions to the Pharmaceutical Benefits Advisory Committee involving economic evaluation. Canberra: Australian Department of Health and Ageing, 2004 Available at: http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-pbs-general-pubs-manual-content.htm-copy2 (accessed Sep 2005).
- 14. Westpac Banking Corporation Ltd. Economics Section. Australian Bureau of Statistics: health and medical services inflation figures. Canberra: Australian Bureau of Statistics, 1999.
- 15. Australian Taxation Office. Guide to depreciation. Canberra; ATO, 1999–2000. Available at: http://www.ato.gov.au/content/downloads/TPRP_Guide_to_Depreciation.pdf (accessed Aug 2005).
- 16. Ware J, Sherbourne C. The MOS 36-item Short-Form Health Survey (SF-36). Med Care 1992; 30: 473-483.
- 17. Martin A, Glasziou P, Simes R. A cardiovascular extension of the Health Measurement Questionnaire. J Epidemiol Community Health 1999; 53: 548-557.
- 18. Martin AJ, Glasziou PP, Simes RJ, Lumley T. Predicting patients’ utilities from quality of life items: an improved scoring system for the UBQ-H. Qual Life Res 1998; 7: 703-711.
- 19. Glasziou PP, Gelber R, Cole BF, et al. Quality adjusted survival analysis with repeated quality of life measures. Stat Med 1998; 17: 1215-1229.
- 20. Department of Health and Ageing website. Glossary. Available at: http://www.health.gov.au/internet/wcms/publishing.nsf/Content/health-pbs-general-pubs-pharmpac-glossary-glossary.htm#u (accessed Aug 2005).
- 21. Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. Boston: Nimrod Press, 1993: 7-10, table 7.4.
- 22. Briggs A, O'Brien B, Blackhouse G. Thinking outside the box: recent advances in the analysis and presentation of uncertainty in cost effectiveness studies. Annu Rev Public Health 2002; 23: 377-401.
- 23. Sundararajan V, Bunker SJ, Begg S, et al. Attendance rates and outcomes of cardiac rehabilitation in Victoria, 1998. Med J Aust 2004; 180: 268-271. <MJA full text>
- 24. Oldridge N, Guyatt G, Jones N, et al. Effects on quality of life with comprehensive rehabilitation after acute myocardial infarction. Am J Cardiol 1991; 67: 1084-1089.
- 25. Ades P, Maloney A, Savage P, Carhart JR. Determinants of physical functioning in coronary patients. Arch Intern Med, 1999; 159: 2357-2360.
- 26. Heller R, Knapp J, Valenti L, Dobson A. Secondary prevention after acute myocardial infarction. Am J Cardiol 1993; 72: 759-762.
- 27. Bergner M, Bobbit R, Carter W, Gilson B. The Sickness Impact Profile: development and final revision of a health status measure. Med Care 1981; 19: 787-805.
- 28. Erdman R, Duivenvoorden H, Verhage F, et al. [Cardiac rehabilitation: a 5-year follow-up study of mental functioning, work resumption, smoking habits and sports activities.] Ned Tijdschr Geneeskd 1984; 128: 846-851. [Dutch]
- 29. Hambrecht R, Niebauer J, Marburger C, et al. Various intensities of leisure time physical activity in patients with coronary artery disease: effects on cardiorespiratory fitness and progression of coronary atherosclerotic lesions. J Am Coll Cardiol 1993; 22: 468-477.
- 30. Ornish D, Brown SE, Scherwitz LW, et al. Can lifestyle changes reverse coronary heart disease? The Lifestyle Heart Trial. Lancet 1990; 336: 129-131.
- 31. Roman O, Gutierrez M, Luksic I, et al. Cardiac rehabilitation after acute myocardial infarction: a 9-year controlled follow-up study. Cardiology 1983; 70: 223-231.
- 32. Sivarajan E, Newton K, Almes M, et al. Limited effects of outpatient teaching and counselling after myocardial infarction: a controlled study. Heart Lung 1983; 12: 65-73.
- 33. Goble A, Hare D, MacDonald PS, et al. Effect of early programmes of high and low intensity exercise on physical performance after transmural myocardial infarction. Br Heart J 1991; 65: 126-131.
- 34. Blumenthal J, Rejeski WJ, Walsh-Riddle M, et al. Comparison of high- and low intensity exercise training early after acute myocardial infarction. Am J Cardiol 1988; 61: 26-30.
- 35. Sivarajan E, Bruce R, Lindskog B, et al. Treadmill responses to early exercise program after myocardial infarction: a randomised study. Circulation 1982; 65: 1420-1428.
- 36. Clausen J. Circulatory adjustments to dynamic exercise and physical training in normal subjects and in patients with coronary artery disease. In: Sonnenblick EH, ed. Exercise and the heart. New York: Grune & Stratton, 1977: 39-75.
- 37. Baigent C, Collins R, Appleby P, et al. ISIS-2: 10 year survival among patients with suspected acute myocardial infarction in randomised comparison of intravenous streptokinase, oral aspirin, both, or neither. The ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. BMJ 1998; 316: 1337-1343.
- 38. Zijlstra F, Patel A, Jones M, et al. Clinical characteristics and outcome of patients with early (< 2 h), intermediate (2-4 h) and late (> 4 h) presentation treated by primary coronary angioplasty or thrombolytic therapy for acute myocardial infarction. Eur Heart J 2002; 23: 550-557.
- 39. George B, Harris A, Mitchell A. Cost-effectiveness analysis and the consistency of decision making: evidence from pharmaceutical reimbursement in Australia (1991-96). Pharmacoeconomics 2001; 19: 1103-1109.
- 40. Scott IA, Lindsay KA, Harden H. Utilisation of outpatient cardiac rehabilitation in Queensland. Med J Aust 2003; 179: 341-345. <MJA full text>





Abstract
Objective: To estimate the incremental effects on cost and quality of life of cardiac rehabilitation after an acute coronary syndrome.
Design: Open randomised controlled trial with 1 year’s follow-up. Analysis was on an intention-to-treat basis.
Setting: Two tertiary hospitals in Sydney.
Intervention: 18 sessions of comprehensive exercise-based outpatient cardiac rehabilitation or conventional care as provided by the treating doctor.
Participants: 113 patients aged 41–75 years who were self-caring and literate in English. Patients with uncompensated heart failure, uncontrolled arrhythmias, severe and symptomatic aortic stenosis or physical impairment were excluded.
Main outcome measures: Costs (hospitalisations, medication use, outpatient visits, investigations, and personal expenses); and measures of quality of life. Incremental cost per quality-adjusted life year (QALY) saved at 1 year (this estimate combines within-study utility effects with reported 1-year risk of survival and treatment effects of rehabilitation on mortality). Sensitivity analyses around a base case estimate included alternative assumptions of no treatment effect on survival, 3 years of treatment effect on survival and variations in utility.
Results: The estimated incremental cost per QALY saved for rehabilitation relative to standard care was $42 535 when modelling included the reported treatment effect on survival. This increased to $70 580 per QALY saved if treatment effect on survival was not included. The results were sensitive to variations in utility and ranged from $19 685 per QALY saved to rehabilitation not being cost-effective.
Conclusions: The effects on quality of life tend to reinforce treatment advantages on survival for patients having postdischarge rehabilitation after an acute coronary syndrome. The estimated base case incremental cost per QALY saved is consistent with those historically accepted by decision making authorities such as the Pharmaceutical Benefits Advisory Committee.