This case describes refeeding syndrome associated with volitional oral nutrition in a patient with chronic alcoholic abuse admitted for detoxification. Refeeding syndrome is an under-recognised and undertreated condition1-5 of severe, acute electrolyte, fluid-balance and metabolic abnormalities in chronically malnourished patients undergoing renutrition.
Refeeding syndrome was first described in Japanese prisoners during World War II.6 Since then, it has been described in patients being refed after hunger strikes, starvation after being lost, chronic alcoholism, anorexia nervosa, malignancy, kwashiorkor and marasmus and in obese patients who have had duodenal switch operations.4,5 Reports conflict over whether it is more common following parenteral7 or enteral tube4 nutrition, but descriptions following volitional oral refeeding are less frequent.
In people in a chronically starved state, insulin secretion is reduced in parallel with low carbohydrate intake. Fat catabolism predominates, and free fatty acids and ketone bodies replace glucose as the major energy source. If starvation is severe, body stores of phosphate, potassium and magnesium may be depleted, although serum levels are often maintained.3-5 With refeeding, there is a shift back to carbohydrate metabolism and an increase in insulin levels. Insulin stimulates the movement of phosphate, potassium and magnesium into the cells, leading to a fall in their serum concentrations.2 In addition, tissue anabolism increases cellular demand for phosphate, glucose, potassium and water.1 Hyperphosphaturia may occur with alcoholism,7,8 and thiamine, required for the intracellular transport of glucose, may be depleted.9
The principal biochemical hallmark of refeeding syndrome, as seen in this case, is severe, acute hypophosphataemia that usually occurs within 3–4 days of refeeding.2,3,10 This is often associated with hypokalaemia, hypomagnesaemia, sodium and fluid retention, thiamine deficiency and hyperglycaemia.
Phosphate is the body’s major intracellular anion.4 Daily oral phosphate intake is about 1000–1400 mg, the major sources being meat, poultry, eggs, cereals and dairy products.4 Wine contains little phosphate.11 Phosphate is found in phospholipids, nucleic acids, adenosine triphosphate and 2,3-diphosphoglycerate in red blood cells. It is important for intracellular buffering, enzymatic phosphorylation, glucose metabolism, nervous system conduction and leucocyte function. Hypophosphataemia-induced depletion of 2,3-diphosphoglycerate in erythrocytes results in a left shift of the haemoglobin/oxygen dissociation curve, increasing haemoglobin affinity for oxygen and predisposing to local tissue hypoxia.1,2,4
However, the clinical manifestations of refeeding syndrome are varied and non-specific (Box 3). Potentially life-threatening sequelae include acute cardiac failure, respiratory failure, Wernicke’s encephalopathy, sepsis and acute renal failure. Sudden cardiac death has been reported in two chronically malnourished patients experiencing acute hypophosphataemia after initiation of total parenteral nutrition.12
Although non-specific, we believe the constellation of symptoms and signs observed in our patient is typical of refeeding syndrome. Most importantly, acute, severe hypophosphataemia not present on admission was noted 4 days after oral refeeding with a ward diet. The presence of a serum phosphate level of 0.15 mmol/L in our patient represents extreme hypophosphataemia. (The lowest published level we are aware of in a patient who survived is 0.07 mmol/L.11) Given the “low normal” value on admission and the patient’s risk factors for refeeding syndrome, the serum phosphate level should have been monitored more closely during the first few days of admission.
We did consider several differential diagnoses. Although hypophosphataemia is commonly seen in sepsis,14 clinical and haematological evidence suggested that the patient’s respiratory infection had largely resolved by Day 4. Acute respiratory alkalosis may also cause hypophosphataemia,15 but was unlikely in this case, in view of the normal serum phosphate level on admission. Severe hypokalaemia was already being corrected by intravenous replacement from the day of admission.
The development of paraesthesiae, myalgias, groin candidiasis, diarrhoea and sinus tachycardia (which may have indicated incipient cardiac failure4,9) was consistent with the diagnosis of refeeding syndrome.4 Unfortunately, the creatinine kinase level was not measured to exclude rhabdomyolysis. Cerebellar signs may have been secondary to alcoholic degeneration or mild Wernicke’s encephalopathy.
Management of refeeding syndrome includes slowing of caloric intake, correcting electrolyte and metabolic abnormalities, monitoring fluid balance and treating complications. Thiamine and B-complex vitamins should be prescribed prophylactically before refeeding.4 Interestingly, the early administration of intramuscular thiamine for chronic alcoholism may have protected our patient against Wernicke’s encephalopathy secondary to refeeding syndrome.
Ideally, patients at risk of developing refeeding syndrome should be identified and a prophylactic low-caloric low-carbohydrate dietary regimen implemented.3 Initially, 85 kJ per kilogram of body weight per day, with a generous protein allowance (1.2–1.5 g protein per kilogram of body weight per day), has been suggested.4,13 Caloric intake can then be gradually increased over the following 1–2 weeks, ensuring that clinical and biochemical parameters are closely monitored.4,12,13 It is important to note that most current recommendations are based on parenteral or enteral tube nutrition. Protocols are not well developed for volitional oral refeeding. However, “slow” refeeding in these patients could be achieved by providing a similar low daily caloric intake with reduced food portions. Ideally, an experienced dietitian should be consulted.4 In our case, a dietitian was not available on site, and, given the prompt correction of electrolyte abnormalities and absence of acute cardiac failure, no change to diet was made.
Levels of serum electrolytes, urea and creatinine should be monitored at least daily in the acute phase. Prophylactic phosphate and potassium supplementation is often required at the time of refeeding in high-risk patients. Phosphate replacement is recommended if serum levels are below 0.3–0.5 mmol/L3,4,10 or if the patient is symptomatic. As oral replacement at these levels is often inadequate, intravenous replacement is advised.7,10 Complications of overzealous intravenous phosphate replacement may include hyperphosphataemia, hypocalcaemia, tetany, hypotension, hyperkalaemia, hypernatraemia, renal failure and metastatic calcification.2,10 Although successful intravenous regimens based on patient weight and serum phosphate levels in intensive care settings have been described,15 these are often complicated and impractical for ward patients.
Terlevich et al10 described the use of 50 mmol intravenous phosphate over 24 hours in 30 ward patients with refeeding syndrome and normal renal function. Twenty-eight patients safely achieved a serum phosphate level above 0.5 mmol/L within 72 hours. We used 42 mmol intravenous phosphate over 36 hours to normalise serum levels in our patient. While less aggressive than the protocol described by Terlevich et al, it was deemed sufficient given that the patient was largely asymptomatic and that serum phosphate levels were improving. Intravenous phosphate was dispensed on site in 14 mmol aliquots, and prescribing this amount over 12 hours simplified the dosing regimen.
Clinical diagnosis of refeeding syndrome requires a high index of suspicion.1 Its hallmark of acute, severe hypophosphataemia in chronically malnourished patients after refeeding may occur even in patients who are largely asymptomatic and orally fed. Prevention of morbidity and, in some cases, death requires careful management of diet, vitamin intake and electrolyte and fluid balance.
1 Serum electrolyte levels over the first 8 days after admission
|
|
Day |
|||||||||||||
Electrolyte |
Reference range |
1 |
4 |
8 |
|||||||||||
Potassium (mmol/L) |
3.6–5.1 |
2.4 |
3.5 |
3.9 |
|||||||||||
Calcium (mmol/L) |
2.25–2.58 |
2.27 |
2.60 |
2.60 |
|||||||||||
Magnesium (mmol/L) |
0.74–1.03 |
0.71 |
0.69 |
0.70 |
|||||||||||
Phosphate (mmol/L) |
0.80–1.50 |
0.84 |
0.15 |
1.56 |
|||||||||||
2 Serum phosphate levels over the first 8 days after admission*
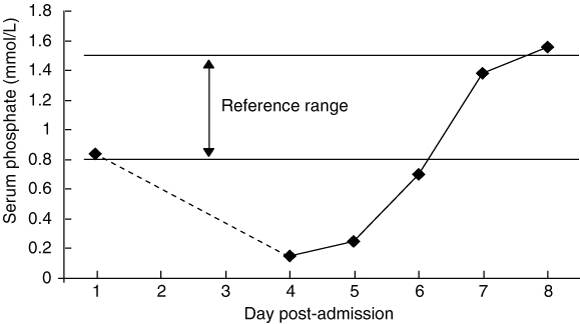
* Dotted line indicates the direction of change only (no data were available for Days 2 and 3).
3 Clinical features of refeeding syndrome4
Clinical feature |
Possible mechanisms |
||||||||||||||
Cardiovascular |
|
||||||||||||||
Acute cardiac failure |
Fluid retention (secondary to carbohydrate intake1,2,4 and hyperinsulinaemia9), arrhythmias, cardiomyopathy1,2,4 |
||||||||||||||
Respiratory |
|
||||||||||||||
Respiratory failure |
Diaphragmatic myopathy2,8 |
||||||||||||||
Neurological |
|
||||||||||||||
Seizures, paraesthesiae |
Electrolyte and/or metabolic disturbance,1,4 cellular hypoxia secondary to reduced 2,3-DPG and ATP |
||||||||||||||
Wernicke’s encephalopathy |
|||||||||||||||
Gastrointestinal |
|
||||||||||||||
Diarrhoea or constipation |
Electrolyte and/or metabolic disturbance,4 intestinal atrophy following malnutrition13 |
||||||||||||||
Haematological |
|
||||||||||||||
Sepsis |
Leukocyte dysfunction, hyperglycaemia, acid–base disturbance1,4 |
||||||||||||||
Haemorrhage |
|||||||||||||||
Haemolytic anaemia |
Depletion of erythrocyte ATP, resulting in increased cell membrane rigidity1 |
||||||||||||||
Metabolic |
|
||||||||||||||
Hyperglycaemia4 |
Glucose ingestion4 |
||||||||||||||
Renal |
|
||||||||||||||
Acute tubular necrosis |
Rhabdomyolysis4 |
||||||||||||||
Musculoskeletal |
|
||||||||||||||
Myopathy |
|||||||||||||||
Rhabdomyolysis |
Impaired production of phospholipid cell membranes causes sarcolemma dysfunction1,2 |
||||||||||||||
ATP = adenosine triphosphate. DPG = diphosphoglycerate. |
|||||||||||||||
- 1. Marinella MA. Refeeding syndrome: implications for the inpatient rehabilitation unit. Am J Phys Med Rehabil 2004; 83: 65-68.
- 2. Marinella MA. The refeeding syndrome and hypophosphataemia. Nutr Rev 2003; 61: 320-323.
- 3. Hearing S. Refeeding syndrome [editorial]. BMJ 2004; 328: 908-909.
- 4. Crook MA, Hally V, Panteli JV. The importance of the refeeding syndrome. Nutrition 2001; 17: 632-637.
- 5. Stubbs C. Case study: refeeding syndrome and thiamin deficiency after extended starvation. Aust J Nutr Diet 1999; 56: 221-223.
- 6. Schnitker MA, Mattman PE, Bliss TL. A clinical study of malnutrition in Japanese prisoners of war. Ann Intern Med 1951; 35: 69-96.
- 7. Maier-Dobersberger T, Lochs H. Enteral supplementation of phosphate does not prevent hypophosphatemia during refeeding of cachectic patients. JPEN J Parenter Enteral Nutr 1994; 18: 182-184.
- 8. Newman JH, Neff TA, Ziporin P. Acute respiratory failure associated with hypophosphatemia. N Engl J Med 1977; 296: 1101-1103.
- 9. Mallet M. Case report: refeeding syndrome. Age Ageing 2002; 31: 65-66.
- 10. Terlevich A, Hearing SD, Woltersdorf WW, et al. Refeeding syndrome: effective and safe treatment with Phosphates Polyfusor. Aliment Pharmacol Ther 2003; 17: 1325-1329.
- 11. Cumming AD, Farquhar JF, Bouchier IA. Refeeding hypophosphataemia in anorexia nervosa and alcoholism. BMJ 1987; 295: 490-491.
- 12. Weinster RL, Krumdieck CL. Death resulting from overzealous total parenteral nutrition: the refeeding syndrome revisited. Am J Clin Nutr 1981; 34: 393-399.
- 13. Faintuch J, Soriano FG, Ladeira JP, et al. Refeeding procedures after 43 days of total fasting. Nutrition 2001; 17: 100-104.
- 14. Sheldon GF. Treatment of hypophosphatemia [letter]. J Am Coll Surg 2004; 199: 171.
- 15. Taylor BE, Huey WY, Buchman TG, et al. Treatment of hypophosphatemia using a protocol based on patient weight and serum phosphorus level in a surgical intensive care unit. J Am Coll Surg 2004; 198: 198-204.





None identified.