An epidemic of chronic disease has arisen among Indigenous people in remote areas of Australia, and renal failure is an important component.1-3 The underlying renal disease is marked by albuminuria, has multiple causes, progresses over time, and marks a greatly increased risk of death from non-renal as well as renal causes.4-7
In late 1995, we introduced a systematic chronic disease treatment program into an Aboriginal community with very high rates of renal failure and deaths from other causes.8 The first 3–4 years of operation of the program have been described previously.9-11 In 2000, responsibility for the program was assumed by the community’s Health Board, after a year-long handover. The Board had been constituted under the Coordinated Care Trials initiative and was funded for primary care services through the federal and territory governments. It retained the same protocols and treatments for chronic disease “activities”, and initially the same Aboriginal staff.
Here, we describe the subsequent evolution of the program and the clinical outcomes to mid-2003.
Treatment was offered to all adults with overt albuminuria (urine albumin–creatinine ratio [ACR] ≥ 34 g/mol), all with diabetes and microalbuminuria (ACR ≥ 3.4 g/mol), and all with blood pressure ≥ 140/90 mmHg. Treatment centred around use of the drug Coversyl (perindopril, a long-acting angiotensin-converting enzyme inhibitor; Servier Laboratories), blood pressure control, and attempts to control blood glucose and lipid levels where appropriate.9-11
Two hundred and sixty-four people were identified as eligible and enrolled in the program.10,11 Their mean age was 43.4 years; 53% were female, 44% had diabetes, 55% had hypertension, 64% had overt albuminuria, and 11% had serum creatinine levels above the laboratory’s reference range. In the first 3 years of the program, two-thirds of the participants reported that they were taking their medicines “some or most of the time”. In the treatment cohort, average blood pressure fell dramatically with treatment, and urine ACR, previously deteriorating, stabilised.5 Average serum creatinine level rose over the first 2 years, and then fell to below baseline values.
At an average of 3.4 years after enrolment, all-cause natural deaths in participants with overt albuminuria had fallen by 50%, and deaths from renal causes had fallen by 57%, when compared with historical controls matched for baseline disease severity.11 Whole-of-community trends in terminal events supported these estimates. Savings in dialysis avoided were estimated at $3.4 million, representing $12 879 per treated person over that period.12,13
The community’s Health Board encountered a series of problems, including deficiencies in the computer systems and a shortfall in anticipated funding, which affected the chronic disease program. The program’s intensity gradually diminished, starting in the last months of the handover and continuing in subsequent years.
There were problems generating lists of people due for rescreening and treatment, diagnoses, and clinical and laboratory results.14 Regular testing of the whole community decreased. Staff roles were reassigned, and the program coordinator left in late 2001 and was not replaced. The discrete chronic disease program was ultimately abandoned, and its activities were incorporated into mainstream care.15
In late 2001, a pharmacy review revealed that only 8% of people prescribed multiple medications were regularly picking up their weekly packs of medicine (Rollo Manning, consultant pharmacist to the Health Board, personal communication). Some concerted action followed, and, in mid to late 2002, medication orders to the supplier suggested that 35%–40% of people prescribed perindopril were taking it with some regularity.
The community’s Health Board went into receivership in September 2003. Responsibilities for health services were then assumed by the Northern Territory Department of Health and Community Services.
Participants in the treatment program were tracked through the community Health Board’s clinical information system and individual medical records, using their unique hospital record numbers. Clinical parameters, documented annually, were followed up to 31 December 2002.14,15 Deaths of program participants and in the community as a whole were documented through community and hospital records to 30 June 2003. All people who started long-term renal dialysis were documented from records of the dialysis unit in Darwin and, more recently, the satellite dialysis unit in the community itself.
A renal “death” was defined as the institution of long-term renal dialysis or death with end-stage renal disease without dialysis.6,7 Non-renal deaths comprised all other deaths from natural causes. Deaths from misadventure, intoxications and self-harm were excluded from the analyses, and participants were censored from the observations when these occurred.
A longitudinal analysis of the treatment cohort was undertaken. Use of contemporaneous controls was rejected, as it would have involved withholding best standard of care from some participants. Use of historical controls, as applied in our earlier analyses,10,11 was no longer possible, as the mean follow-up of the treatment cohort (with an extra 3 years) now vastly exceeded the follow-up of controls in the untreated state.
Average blood pressure levels and the proportions of participants with heavy albuminuria (ACR ≥ 100 g/mol) and raised serum creatinine levels (men, ≥ 120μmol/L; women, > 102 μmol/L) were calculated for annual intervals after enrolment. Because enrolment into the program was staggered, the numbers of people contributing to each observation fell as the interval since enrolment increased. Calculations were repeated for the 127 people who were followed up for 6 years or more after enrolment. Rates of terminal events were calculated as events/total person-years at risk. Cox regression analyses were used to calculate hazard ratios for terminal events in the remaining treatment cohort for the period 1 January 2002 to 30 June 2003, compared with terminal events in participants in the first 18 months after their enrolment in the systematic phase of the program. These hazard ratios were adjusted for sex, age and baseline urine ACR, which are strong predictors of terminal outcomes.6,7
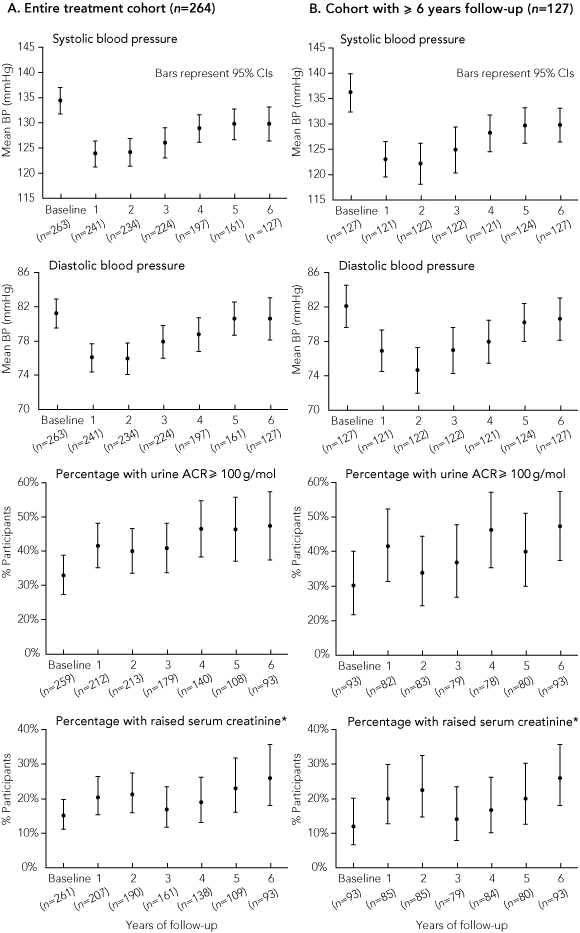
Blood pressure and renal markers: Box 1A shows trends in blood pressure and renal function at annual intervals after enrolment in the participants remaining in the cohort. Average blood pressure began to rise 3 years after enrolment, followed by deteriorations in ACR and serum creatinine level 1–2 years later. Box 1B shows similar trends in the cohort of 127 people who were followed up for at least 6 years.
Renal and non-renal deaths: There were 49 renal and non-renal natural deaths in the treatment cohort over a total observation period of 1539 person-years: 20 people entered long-term dialysis, two died of terminal chronic renal failure without undergoing dialysis, and 27 died of non-renal natural causes.
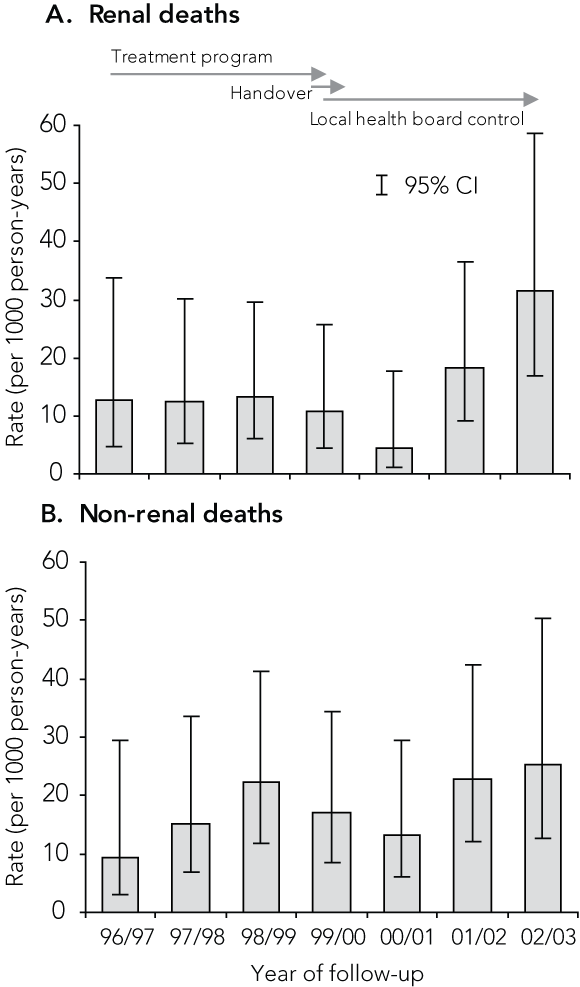
Box 2 shows death rates over sequential intervals. For the first five intervals, renal death rates remained suppressed, at a rate about 60% lower than in matched controls in the pre-treatment era.7,8,10,11 However, rates increased in the last two intervals (2001–2002 and 2002–2003). Trends in non-renal natural deaths were not so clear, although the rate in the last interval was the highest in the observation period.
Box 3 shows that the risk of all-cause natural deaths (renal plus non-renal) in people remaining in the treatment cohort in 2002–2003 was 4.3 times the risk within the first 18 months of starting treatment. The risk of renal death was increased three-fold and the risk for non-renal death was increased 9.5-fold.
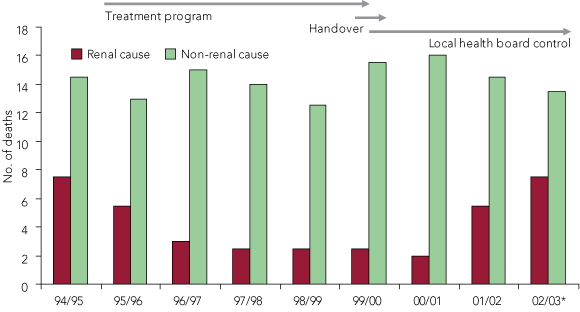
Box 4 shows the numbers of renal and non-renal deaths in the community as a whole, regardless of participation in the treatment program. It reflects the improvement during the systematic phases of the program, and the deterioration in the last two observation intervals. Almost 75% of the renal deaths after the handover of the program were among people in the treatment cohort. Trends in non-renal deaths were not definitive.
Eighty per cent of the people in the community who developed renal failure entered the dialysis program. Thus, the reduction in terminal events during the more active phase of the treatment program and the resurgence in the last few years had major impacts on the numbers of people in the end-stage renal disease treatment program and the costs of running it.
In this high-risk community, systematic treatment of renal disease and hypertension produced clinical benefit and reduced renal and non-renal deaths. Diminished intensity of the program was associated with deterioration in blood pressure and kidney function, and ultimately increases in rates of renal failure and non-renal deaths. The deterioration in outcomes followed the marked reduction in compliance with medication documented after the handover, although regression to the mean and ageing of the treatment cohort could also have contributed.
Thus, vigorous treatment of people with morbidities must be sustained. Although treatment retards disease progression, it does not eliminate risk.
The resurgence of renal failure and deaths has serious impacts on suffering, family and community dynamics, and health care costs. However, incentives for community services to reduce these costs are not powerful when primary care is disassociated (conceptually, operationally and fiscally) from hospital and renal dialysis and transplantation services, and when the societal costs of premature death in Aboriginal adults, especially in remote settings, are not assigned a value.
The events reported here, together with our experiences in a chronic disease outreach program in five other remote settings, suggest some ingredients that contribute to a successful and sustained program.16,17 There needs to be a commitment to ongoing chronic disease surveillance, treatment and education, as an essential and major element of primary care. Activities must be adequately and reliably funded and staffed over the long term. When health care funding and staff are spread thinly, activities perceived to be elective (eg, health promotion, mental health and chronic disease care) are the first to be pared in the face of further constraints or crises. An informed and supportive administration and a specifically trained Indigenous work force, which is allocated significant roles, respected and properly paid, are also essential.16-21 The clinical environment should be welcoming and respectful, regardless of the extent to which people can comply with lifestyle and treatment recommendations.
In addition, treatment decisions should be simplified and expedited,22,23 using a restricted number of drugs in each class, once-a-day dosing whenever possible, and easily transportable multidrug blister packs. Home visits and reminder systems based on daily events could improve medicine taking. However, there is serious need for innovation, such as a range of therapeutic dose “polypills”24 and transdermal drug delivery systems.
It is important to keep abreast of treatment advances. Better outcomes can now be expected from target blood pressures ≤ 125/75 mmHg in people with albuminuria, combination treatment with angiotensin-converting enzyme inhibitors and angiotensin-blockers,25,26 vigorous lipid-lowering therapy, better glucose control (often with added long-acting insulin), and more widespread use of aspirin.
Finally, a good clinical information system is needed to schedule visits, to follow clinical and laboratory results, to document process measures, outcomes and trends, to create community health profiles, to estimate cost effectiveness, and to assess the need for program modifications.13,21,27
In the past 2 years, chronic disease management has been reinvigorated in this community. The medical workforce has increased (at least for a time), systematic testing of the population has been re-established with appropriate management referrals, and, on a more recent audit, about 70% of prescribed medication was being picked up from the pharmacy. These events promise some regression of the current resurgence in renal morbidity and mortality.
Titrating management to a level of excellence and maintaining vigorous program activity remain ongoing challenges.
3 Renal and non-renal deaths in the treatment cohort in the final 18-month observation period (January 2002 to June 2003) compared with deaths in the cohort in the initial 18 months after enrolment in the treatment program*
|
No. deaths |
Rate per 1000 person-years (95% CI) |
P† |
Unadjusted hazard |
P‡ |
Adjusted hazard ratio (95% CI)§ |
P‡ |
||||||||
|
Initial |
Final |
Initial |
Final |
|||||||||||
All-cause natural deaths |
5 |
18 |
13.3 (5.5–31.8) |
56.7 (35.7–90.0) |
0.002 |
4.3 (1.6–11.5) |
0.0004 |
4.9 (1.8–13.6) |
0.002 |
||||||
Renal deaths |
4 |
10 |
10.6 (4.0–28.3) |
31.5 (17.0–58.6) |
0.05 |
3.0 (0.9–9.5) |
0.07 |
4.6 (1.4–15.0) |
0.01 |
||||||
Non-renal deaths |
1 |
8 |
2.7 (0.4–18.8) |
25.2 (12.6–50.4) |
0.009 |
9.5 (1.2–75.8) |
0.03 |
7.3 (0.9–59.5) |
0.06 |
||||||
* Initial 18-month period was not a specific calendar period as enrolment was staggered from 1996. There were 377.3 person-years at risk in the initial period and 317.4 in 2002–2003. †Log-rank test for significance of difference between death rates. ‡Wald test for significance of hazard ratios. §Adjusted for sex, age and baseline urine albumin–creatinine ratio. |
|||||||||||||||
- Wendy E Hoy1
- Srinivas N Kondalsamy-Chennakesavan2
- Jennifer L Nicol3
- Centre for Chronic Disease, University of Queensland, Brisbane, QLD.
This manuscript is dedicated with respect and gratitude to the late Mr Barry Young, OAM, FRACP, former General Manager of Servier Australia, for his moral support, and for the resources donated to the treatment program. Without these, none of this work, nor its derivative programs in Australia and internationally, would have occurred.
The treatment program and its evaluation were supported by Servier Australia, the National Health and Medical Research Council, the Australian Kidney Foundation (now Kidney Health Australia) and Rio Tinto. The follow-up study was supported by the Cooperative Research Centre for Aboriginal Health, the Office of Aboriginal and Torres Strait Islander Health (OATSIH), and the Colonial Foundation of Australia.
We are grateful for the support and participation of the members of this community, their Land Council and their Health Board. We especially thank all the health workers, as well as the nursing staff and the resident and visiting medical officers and specialists serving this community. We thank Dr Gary Robinson (Charles Darwin University, Darwin, NT) for his comments and suggestions.
The observations in this article were made in projects approved by the Ethics Committee of the Royal Darwin Hospital and the Menzies School of Health Research and by the Community’s Land Council and Health Board as follows: “The epidemiology and prevention of Aboriginal renal disease, part 1” (1992); “The epidemiology and prevention of Aboriginal renal disease, part 2” (1994); “A follow-up study of Coordinated Care Trial renal treatment outcomes” approved by the Northern Territory University (H01010) and the Menzies School of Health Research (01/22), and “An updated view of chronic disease profiles and chronic disease deaths in a remote Aboriginal Community: a ten year follow-up” (2003). The last has also been approved by the Human Research Ethics Committee of the University of Queensland and the Royal Brisbane Hospital.
None identified.
- 1. Spencer JL, Silva DT, Snelling P, Hoy WE. An epidemic of renal failure among Australian Aboriginals. Med J Aust 1998; 168: 537-541. <MJA full text>
- 2. McDonald SP, Russ GR. Current incidence, treatment patterns and outcome of end-stage renal disease among indigenous groups in Australia and New Zealand. Nephrology (Carlton) 2003; 8: 42-48.
- 3. Australian Bureau of Statistics. The health and welfare of Australia’s Aboriginal and Torres Strait Islander peoples. Canberra: ABS, 2001. (ABS Catalogue No. 4704.0.)
- 4. Hoy WE, Mathews JD, McCredie DA, et al. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int 1998; 54: 1296-1304.
- 5. Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 1. Changes in albuminuria and glomerular filtration rate over time. Kidney Int 2001; 60: 243-248.
- 6. McDonald SP, Wang Z, Hoy WE. Physical and biochemical predictors of death in an Australian aboriginal cohort. Clin Exp Pharmacol Physiol 1999; 26: 618-621.
- 7. Hoy WE, Wang Z, VanBuynder P, et al. The natural history of renal disease in Australian Aborigines. Part 2. Albuminuria predicts natural death and renal failure. Kidney Int 2001; 60: 249-256.
- 8. Hoy WE, McFarlane R, Pugsley DJ, et al. Markers for cardiovascular and renal morbidity: expectations for an intervention programme in an Australian aboriginal community. Clin Exp Pharmacol Physiol 1996; 23(8): S33-S37.
- 9. Hoy WE, Kelly A, Jacups S, McKendry K, et al. Stemming the tide: reducing cardiovascular disease and renal failure in Australian Aborigines. Aust N Z J Med 1999; 29: 480-483.
- 10. Hoy WE, Baker PR, Kelly AM, Wang Z. Reducing premature death and renal failure in Australian Aboriginals. A community-based cardiovascular and renal protective program. Med J Aust 2000; 172: 473-478. <MJA full text>
- 11. Hoy WE, Wang Z, Baker PR, Kelly AM. Reduction in natural death and renal failure from a systematic screening and treatment program in an Australian Aboriginal community. Kidney Int Suppl 2003; 83: S66-S73.
- 12. You J, Hoy WE, Zhao Y, et al. End-stage renal disease in the Northern Territory: current and future treatment costs. Med J Aust 2002; 176: 461-465. <MJA full text>
- 13. Baker PR, Hoy WE, Thomas RE. Cost-effectiveness analysis of a kidney and cardiovascular disease treatment program in an Australian Aboriginal population. Adv Chronic Kidney Dis 2005; 12: 22-31.
- 14. Kondalsamy-Chennakesavan S. Sustaining renal health outcomes following a community-based intervention program [MPH thesis]. Darwin: Northern Territory University, 2003.
- 15. Robinson GB, Wang Z, Snelling P, Kondalsamy-Chennakesavan S. A follow-up study of outcomes of the Tiwi renal treatment program. Darwin: Northern Territory University, 2003.
- 16. Hoy WE, Kondalsamy-Chennakesavan S, Scheppingen J, Sharma S, McKendry K. Final report on the Aboriginal Chronic Disease Outreach Program, submitted to Kidney Health Australia and the Office of Aboriginal and Torres Strait Islander Health. Brisbane: Centre for Chronic Disease, University of Queensland, 2003.
- 17. Hoy WE, Kondalsamy-Chennakesavan S, Scheppingen J, Sharma S. Kidney and related chronic disease profiles and risk factors in three remote Australian Aboriginal communities. Adv Chronic Kidney Dis 2005; 12: 64-70.
- 18. Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med 1997; 127: 1097-1102.
- 19. Kotter JP. What leaders really do. Harvard Business Review 1990; 68(3): 103-111.
- 20. Davey RG, Gokel G, Hoy WE. Obstacles to good management of chronic disease in remote Aboriginal Australia. Nephrology 2002; 7 Suppl: A78.
- 21. Hoy WE, Kondalsamy-Chennakesavan S, Scheppingen J. Western Australian Chronic Disease Outreach Program. Annual report for the year 2004. Brisbane: Centre for Chronic Disease, University of Queensland, 2005.
- 22. Hoy WE, Chennakesavan Kondalsamy S. A simplified approach to renal and cardiovascular protection for Aboriginal people with type 2 diabetes [abstract of poster]. Nephrology (Carlton) 2004; 9 Suppl 1: 3A80.
- 23. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med 2000, 342: 145-153.
- 24. Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80%. BMJ 2003; 326: 1419.
- 25. Nakao N, Yoshimura A, Morita H, et al. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet 2003; 361: 117-124.
- 26. Kincaid-Smith P, Fairley K, Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant 2002; 17: 597-601.
- 27. Wagner EH, Davis C, Schaefer J, et al. A survey of leading chronic disease management programs: are they consistent with the literature? Manag Care Q 1999; 7: 56-66.





Abstract
In late 1995, a treatment program for renal disease and hypertension was introduced into a remote Aboriginal community. Over the next 3.5 years, mean blood pressure levels were markedly reduced, renal function stabilised, and rates of both renal and non-renal deaths declined significantly.
In 1999–2000, responsibility for the program was passed to the community’s local Health Board, which subsequently faced deficiencies in clinical information systems and a shortfall in funding.
After the handover, the intensity of the program declined, and compliance with medicines fell. Blood pressures in the treatment cohort increased, renal function deteriorated, and rates of deaths from natural causes subsequently rose. From 2002 to mid-2003, the adjusted risks of renal and non-renal deaths in the treatment cohort were three and 9.5 times the respective risks of people during the first 18 months of treatment in the systematic phase of the program.
Sustained vigorous activity, both in treatment of people already identified and in community screening for treatment eligibility, is required to maintain good results in any chronic disease program. Adequate resources and well supported staff are essential, and constant evaluation is needed to follow outcomes and modify strategies as necessary.