Pethidine is not the opioid analgesic of choice for patients in hospital emergency departments (EDs),1 and Australian guidelines describe the limitations of pethidine as an analgesic (Box 1).1-6 Pethidine should not be used as first-line treatment for acute pain, and should only be used “for very brief courses in otherwise healthy patients who have demonstrated an unusual reaction . . . or allergic response during treatment with other opioids”.7
Despite its limitations and the lack of good evidence to support using it preferentially,8 pethidine continues to be widely prescribed in hospitals. In EDs, where patients may have multiple clinical problems and where a detailed medication history may not be available, other opioid analgesics are safer. Nevertheless, a survey of 18 New South Wales hospitals in 2001 showed that up to 38% of pethidine prescribing occurred in EDs (NSW Therapeutic Advisory Group, unpublished data). We therefore set out to promote evidence-based analgesic choice and reduce pethidine prescribing in NSW public hospital EDs.
Drug use evaluation (DUE) is an established method for changing prescribing practice.9,10 It uses an iterative quality cycle10,11 and measures success by comparing observed practice with agreed standards, such as evidence-based guidelines. DUE methods (Box 2) are similar to plan-do-study-act (PDSA) quality improvement methods, and were developed to monitor and influence prescribing practices.11 We applied DUE methods in a multicentre model to influence pethidine prescribing practices over 12 months.
All hospitals in the NSW Therapeutic Advisory Group (NSW TAG) network were invited to participate. The NSW TAG network represents Drug and Therapeutics Committees (DTCs) from public hospitals in NSW. All principal referral, paediatric specialist, major metropolitan and major non-metropolitan public hospitals with a DTC are included in the NSW TAG network, as well as some district hospitals. At the time we conducted this study, there were 46 hospitals represented in the network.
All NSW TAG hospitals that participated were considered “intervention” hospitals. “Control” hospitals comprised 12 non-participating NSW TAG hospitals from which analgesic distribution and ED presentations data were also requested.
A project coordinator in each participating hospital coordinated local data collection, feedback and education programs. Hospital coordinators attended a workshop where recognised local experts and opinion leaders provided educational sessions on pain management, DUE methods, social marketing techniques and change management. Hospitals received no financial support for participating in the project.
Approval was obtained from the Institutional Ethics Committee, Drug and Therapeutics Committee and Chief Executive Officer in each hospital.
Participating hospitals were coded for aggregated reporting and classified according to the Australian Institute of Health and Welfare (AIHW) peer group classification.12
Three DUE cycles were implemented, involving collection of audit data, evaluation of data against pre-determined standards (existing national and local guidelines1-6), feedback of evaluated data, and targeted interventions. Interventions were based on published literature,9 and included use of educational materials, audit and feedback, facilitated small-group discussions and advice from local opinion leaders. Each hospital coordinator selected and implemented a range of interventions, informed by local audit results.
We examined data for the 12 months from 1 September 2002 to 31 August 2003 and continued to collect data until February 2004. Educational interventions commenced in December 2002. One-week prescribing audits were undertaken in January, April and July 2003. Drug register records were used to identify pethidine prescriptions during audit periods. (Under NSW legislation, the dispensing and/or administration of all drugs of dependence must be recorded in a drug register.) Details for each prescribing episode were recorded by ED staff. The number of patient presentations to the ED was recorded monthly. Data were de-identified.
Distribution of parenteral analgesics (pethidine, morphine, tramadol, fentanyl and ketorolac) from pharmacy to the ED and other wards was used to monitor analgesic use. As pethidine, morphine and fentanyl are accountable drugs, distribution figures were considered to correlate closely with prescribing.
Data were recorded monthly and aggregated in 3-month blocks. Data from “control” hospitals were collected retrospectively and aggregated in 3-month blocks. Epi Info (version 6; Centers for Disease Control and Prevention, Atlanta, Ga, USA) was used to calculate the mid-point 95% confidence intervals for proportions and χ2 test for statistical comparison of proportions.
For feedback to prescribers, data were entered into an Excel 2000 file template linked to a PowerPoint 2000 presentation (Microsoft Corporation, USA). Hospital coordinators presented feedback reports to ED staff and the hospital DTC after each audit. Aggregated hospital data were reported to allow cross-comparison within hospital peer groups.
Prescribing was compared with evidence-based guidelines. Non-concordance was identified and used to inform targeted educational interventions. NSW TAG coordinated the preparation of materials to support hospital coordinators, including copies of guidelines, slide presentations, posters and bookmarks. Posters and bookmarks reiterated the messages in Box 1 and provided guidance for use of alternative analgesics in common indications. Opinion leaders’ answers to frequently asked questions about pethidine and pain management in the ED were collated and distributed to hospital coordinators to assist in small group discussions. All resources were made available via the NSW TAG website <http://www.nswtag.org.au>.
Forty-six NSW public hospitals were invited to participate; 22 accepted. An additional Victorian hospital was also included. There were seven principal referral hospitals (AIHW peer group A12), six major non-teaching hospitals (group B) and 10 district or community hospitals (groups C/D). Reasons for non-participation included: inadequate staffing (4), low usage of pethidine in the ED (3), concurrent ED projects (1) and preference for immediate policy restriction (1); 15 gave no reason. Twelve non-participating hospitals also provided drug distribution data (6 group A, 4 group B and 2 group C/D hospitals).
Prescription audits identified the indications for which pethidine was prescribed in participating hospitals over the three DUE cycles. These are presented in Box 3.
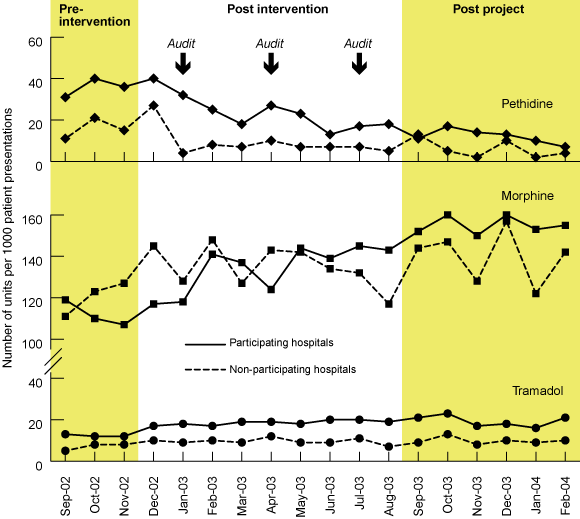
In the 12 months between the pre-intervention period and the equivalent post-intervention period, pethidine use in EDs (as measured by the absolute number of dosage units issued in each 3-month period) decreased by 62% in project hospitals (95% CI, 60%–63%; 4669 to 1793 units) compared with 56% in non-participating hospitals (95% CI, 54%–59%; 1476 to 648 units; P < 0.001). Six months after project completion, pethidine use in project hospitals had decreased from baseline by 71% (95% CI, 70%–72%; 4669 to 1348 units) and in non-participating hospitals by 64% (95%CI, 62%–66%; 1476 to 532 units; P < 0.001).
Trends in ED analgesic use were independent of the number of patients presenting to EDs (Box 4).
There was a 47% increase from baseline in the use of morphine in participating EDs over the 18-month period (95% CI, 46%–48%;14 659 to 21 555 units) and a corresponding increase of 22% in non-participating hospitals (95% CI, 22%–23%; 11 424 to 13 979 units). During the same period, there was a 53% increase from baseline in the use of tramadol in participating EDs (95% CI, 50%–55%; 1623 to 2476 units), and a corresponding 39% increase in non-participating hospitals (95% CI, 35%–43%; 686 to 952 units). Use of both ketorolac and fentanyl remained minimal throughout the project.
In participating hospitals, there was a 51% reduction in pethidine use (95% CI, 50%–51%; 18 632 to 9190 units) in hospital departments other than EDs over the same 18-month period. This reduction was significantly smaller than that observed in these hospitals’ EDs (P < 0.001).
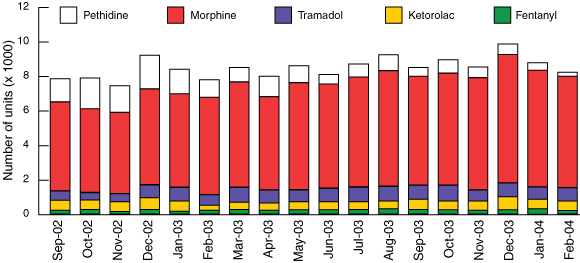
The overall use of parenteral analgesics was monitored in participating hospitals to ensure that patients were not being deprived of effective analgesia. The total number of units of parenteral analgesics issued increased from baseline by 16% (95% CI, 16%–17%; 23 692 to 27 486) 6 months after project completion (Box 5).
Pethidine prescribing in EDs in NSW public hospitals decreased over the course of this project, but the reasons for the decrease are unclear.
One purpose of quality improvement methods is to establish a relationship between process or system changes and variation in outcomes. Our project failed to show a clear temporal relationship between interventions and results. In EDs there is typically a time-lag between issue of stock from pharmacy and actual use, which may delay evidence of change by up to 4 weeks. Future needs may also be anticipated inconsistently, and more or less stock than is actually used may be requisitioned. Temporal relationships are therefore difficult to interpret. Routine monitoring of actual prescribing data rather than stock distribution data would have overcome these deficiencies, but was not feasible in our study.
In an attempt to control for external factors, we collected data retrospectively from non-participating hospitals in our network. The reduction in pethidine use in these hospitals could be explained by the fact that key messages were widely promulgated throughout the NSW TAG network, and no attempt was made to segregate participating and non-participating hospitals. Pre-existing low prescribing rates may explain the smaller reduction over time in non-participating hospitals compared with participating hospitals. There were also fewer district or community hospitals (groups C or D) in the non-participating group (two compared with 10 in participating hospitals), which limited comparison between groups.
Non-ED wards in participating hospitals showed a downward trend in pethidine use, which was significantly smaller than the trend observed in EDs. However, we cannot discount the possibility of a pre-existing trend toward reduced pethidine use, which would have continued in the absence of our intervention.
Time-series analysis may have provided a more rigorous assessment of the impact of our interventions. However, this technique requires confidence in the use of baseline as historical control.13 We used a baseline period of only 3 months before intervention, and pethidine use was not stable during this period. We therefore cannot discount the possibility that other external factors were exerting influences on prescribing practices. Much longer baseline and follow-up periods would have been desirable, but were precluded by the time limitations of project funding.
A number of external factors may have influenced analgesic prescribing practices in EDs during the project. There were several concurrent collaborative projects in progress involving Australian EDs, including the National Institute of Clinical Studies ED Collaborative, and the Australian Council for Safety and Quality in Health Care Medication Safety Breakthrough Collaborative. Some participating hospitals were involved in these collaboratives, and some were focusing on issues related to analgesia in EDs (although not specifically pethidine prescribing).
Tramadol was marketed in Australia in 1998 and now provides an alternative to traditional opioids for management of moderate pain. A trend away from pethidine use and toward tramadol use may have pre-dated this project. Tramadol is associated with potentially severe adverse reactions, including seizures6 and serotonin syndrome,8,14 and with many drug–drug interactions. It therefore may not be a safer alternative to pethidine in this setting.
Interventions were not uniformly implemented in all project hospitals, which was not unexpected given the differences in hospital complexity and available staff resources.15 All hospital coordinators distributed educational materials, provided feedback on departmental prescribing and facilitated at least one small group discussion in each cycle. None provided academic detailing (educational outreach visits) to individual prescribers. While multifaceted interventions were encouraged, coordinators reported that a lack of dedicated project staff influenced their team’s ability to implement multiple interventions in each cycle. Successful hospitals reported including local opinion leaders in their activities, a strategy recognised as important in influencing prescribers.16,17 Some hospitals used the project as the impetus to formally change prescribing policies in their EDs. Four hospitals removed pethidine from the hospital formulary at the end of the project. These hospitals reported that the project helped educate prescribers about policy changes and facilitated support for policy implementation.
Whether reduced pethidine prescribing in NSW hospitals is actually associated with better patient outcomes is not known. There is currently no mechanism in hospitals for systematic monitoring of all adverse drug reactions associated with medicines use. Reporting of adverse reactions to the Australian Drug Reactions Advisory Committee (ADRAC) is encouraged, but is voluntary, and focuses on new drugs or unusual reactions. It does not provide a mechanism for monitoring safety improvements over time. Hospital-based adverse drug reaction reporting programs have similar limitations.
There are good theoretical reasons for encouraging use of morphine as the opioid of choice and for discouraging use of pethidine in patients in the ED setting. This study showed that there was a sustained reduction in pethidine use in hospital EDs between September 2002 and February 2004, which may indicate successful promotion of safer analgesic prescribing. It is not clear whether changes were a result of collaborative DUE methods or other factors.
Shorter duration of action than morphine with no additional analgesic benefit.
Similar side effects to morphine, including bronchospasm and increased biliary pressure.
Metabolised to norpethidine which has potential toxic effects (eg, convulsions) especially in renal dysfunction.
Associated with potentially serious interactions with other drugs, including mono-amine oxidase inhibitors and serotonin reuptake inhibitors, which may result in serotonin syndrome.8
Pethidine is the drug most commonly requested by drug abusers seeking opioids and the drug most often abused by health professionals.
2 Drug use evaluation methods
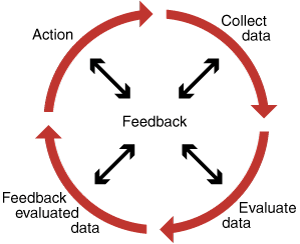
Reproduced (with permission) from The Society of Hospital Pharmacists of Australia Drug Usage Evaluation Starter Kit, Melbourne, 1998.
3 Indications for pethidine use documented by emergency department staff during the three audit periods (participating hospitals only)
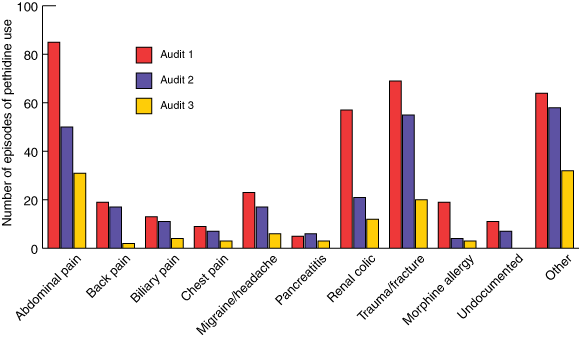
Note: Subsequent educational messages targeted common indications (eg, abdominal pain, renal colic, migraine).
Received 16 July 2004, accepted 23 June 2005
- Karen I Kaye1
- Susan A Welch2
- Sharon R Davis3
- Linda V Graudins4
- Andis Graudins5
- Tai Rotem6
- Richard O Day7
- 1 NSW Therapeutic Advisory Group Inc, Sydney, NSW.
- 2 Prince of Wales Hospital.
- 3 School of Public Health and Community Medicine, University of New South Wales, Sydney, NSW.
- 4 Clinical Pharmacology and Toxicology, St Vincent's Hospital, Sydney, NSW.
NSW TAG gratefully acknowledges funding provided by the National Institute of Clinical Studies (NICS) which allowed this project to be undertaken. We also thank: Associate Professor Milton Cohen (Pain Physician), Mr Stuart Dorkins (Emergency Nurse), Dr Robert Dowsett (Emergency Physician), Ms Kanan Gandecha (Pharmaceutical Services Branch, NSW Health Department), Ms Margaret Knight (Consumer), Ms Kathleen Ryan (Quality Manager), Dr Jan Davies (NICS), Ms Sue Huckson (NICS) and Dr Alex Wodak (Alcohol and Drug Specialist), members of the Steering Committee for willing commitment and expert guidance. We are indebted to hospital project coordinators and emergency department teams at Auburn, Bankstown, Batemans Bay, Bega, Blacktown, Cooma, Frankston, Goulburn Base, Grafton Base, John Hunter, Lismore Base, Moruya, Mt Druitt, Mullumbimby, Murwillumbah, Prince of Wales, Queanbeyan, Royal North Shore, Royal Prince Alfred (Sydney), Sydney Eye, Westmead, Wollongong and Young hospitals. We thank members of the NSW TAG network for their support and Mr David Maxwell for helpful advice on the manuscript.
None identified.
- 1. National Health and Medical Research Council. Acute pain management: scientific evidence. Canberra: NHMRC, 1999.
- 2. NSW Therapeutic Assessment Group. Prescribing guidelines for primary care clinicians. Rational use of opioids in chronic or recurrent non-malignant pain: migraine. Sydney: NSW TAG, 2002.
- 3. NSW Therapeutic Assessment Group. Prescribing guidelines for primary care clinicians. Rational use of opioids in chronic or recurrent non-malignant pain: low back pain. Sydney: NSW TAG, 2002.
- 4. NSW Therapeutic Assessment Group. Prescribing guidelines for primary care clinicians. Rational use of opioids in chronic or recurrent non-malignant pain: general principles. Sydney: NSW TAG, 2002.
- 5. Mashford ML, Cohen ML, Collin H, et al. Therapeutic guidelines: analgesic. 4th ed. Melbourne: Therapeutic Guidelines Limited, 2002.
- 6. Australian medicines handbook. Adelaide: Australian Medicines Handbook Pty Ltd, 2004.
- 7. Agency for Health Care Policy and Research (AHCPR). Acute pain management: operative or medical procedures and trauma. Clinical Practice Guideline Number 1. Rockville, MD: AHCPR, 1992. (AHCPR Publication No. 92-0032.)
- 8. Latta KS, Ginsberg B, Barkin RL. Meperidine: a critical review. Am J Ther 2002; 9: 53-68.
- 9. Campbell D, Scott I, Anderson J, Greenberg P. Improving clinical practice: what works and what doesn’t? Intern Med J 2001; 31: 536-540.
- 10. Dartnell JGA, Kirsa SW. DUE — an essential tool to achieve QUM [editorial]. Aust J Hospital Pharmacy 2000; 30: 251-252.
- 11. Dartnell JGA. Understanding, influencing and evaluating drug use. Melbourne: Therapeutic Guidelines Limited, 2001.
- 12. Australian Institute of Health and Welfare (AIHW). Australian hospital statistics 2001–02 (Table A4.2). Health Services Series No. 20. Canberra: AIHW, 2002. (AIHW Catalogue No. HSE 25 2003.)
- 13. Speroff T, O’Connor GT. Study designs for PDSA quality improvement research. Q Manage Health Care 2004; 13: 17-32.
- 14. Adverse Drug Reactions Advisory Committee. Tramadol – four years’ experience. Aust Adv Drug React Bull 2003; 22: 1.
- 15. Feder G, Eccles M, Grol R, et al. Clinical guidelines: using clinical guidelines. BMJ 1999; 318: 728-730.
- 16. Lomas J, Enkin M, Anderson G, et al. Opinion leaders vs audit and feedback to implement practice guidelines. Delivery after previous cesarean section. JAMA 1991; 265: 2202-2207.
- 17. Scott I, Buckmaster N, Harvey K. Clinical practice guidelines: perspectives of clinicians in Queensland public hospitals. Intern Med J 2003; 33: 273-279.





Abstract
Objective: To reduce pethidine prescribing in hospital emergency departments (EDs).
Design: Multi-centre drug use evaluation (DUE) process.
Setting and participants: Emergency departments in 23 public hospitals (22 in New South Wales, 1 in Victoria) from 1 September 2002 to 31 August 2003. Participating hospitals included seven principal referral hospitals, six major non-teaching hospitals and 10 district or community hospitals. Data for comparison were collected from 12 non-participating hospitals.
Interventions: Hospital coordinators at each participating hospital were provided with support to implement a range of prescribing interventions in their ED in each of three DUE cycles. Interventions included educational materials (guidelines, posters, prescribing reminders), audit and feedback, and small-group discussions. Three audits of pethidine prescribing were undertaken. Prescribing was compared with evidence-based guidelines and non-concordance identified.
Main outcome measures: Number of dosage units of parenteral analgesics issued to the ED from each hospital’s pharmacy department was recorded monthly and aggregated in 3-month periods.
Results: In the 12 months between the preintervention period and the equivalent post-intervention period, pethidine use decreased by 62% in project hospitals (4669 to 1793 units) and 56% in control hospitals (1476 to 648 units). Six months after project completion there was a significantly greater reduction from baseline in participating hospitals (71%; 4669 to 1348 units) compared with non-participating hospitals (64%; 1476 to 532 units; P < 0.001). There was a concurrent increase in use of both morphine and tramadol.
Conclusion: There was a sustained reduction in pethidine use during the study period, which may indicate successful promotion of safer analgesic prescribing. It is not clear whether changes were a result of collaborative DUE methods or other factors.