We recently diagnosed rickettsial spotted fever in four patients from the south-eastern coastal region of South Australia near Adelaide, an area not known to be endemic for this infection. All infections were acquired within the geographic range of Aponomma hydrosauri, the tick vector of Rickettsia honei. Infection by R. honei was confirmed in two patients. This extension of the known geographic range of R. honei infection may be explained, in part, by alterations in host–parasite ecology.
Two spotted fever group (SFG) rickettsia species endemic to Australia are known human pathogens — Rickettsia australis and Rickettsia honei.1 R. australis, the agent of Queensland tick typhus (QTT), is transmitted by ticks of the genus Ixodes, principally I. holocyclus,2,3 and has been associated with infections along the eastern seaboard from tropical north Queensland to Wilsons Promontory, in Victoria. The hosts for these tick vectors are mammals, including native rats and bandicoots.2 Confirmed cases of R. honei infection, or Flinders Island spotted fever, have previously been described only on Flinders Island, in Bass Strait,4 although a rickettsial spotted fever illness, possibly caused by R. honei, has also recently been reported from the east coast of Tasmania.5 Recently published findings indicate that the parasitic tick Aponomma hydrosauri, which has a variety of reptile species as its hosts, may be the principal vector of R. honei.6
We describe four patients from coastal South Australia (SA) near Adelaide who presented with an illness characterised by fever, severe malaise, diffuse maculopapular rash, and laboratory findings indicating acute SFG rickettsial infection.
In October 2001, a 65-year-old woman from the south coast of SA (Box 1) was referred after 5 days of high fever, rigors, prostration, headache and generalised muscle pain. On Day 5, a non-pruritic rash appeared on the trunk and subsequently spread to the extremities, including the palms and soles. Two days before the onset of fever, while working in her garden, she had experienced sudden pain in her right groin, which she attributed to an ant bite; no tick or other arthropod was seen. She developed a mild purple skin discoloration in her right groin, but no eschar. She had not travelled recently or been bushwalking and had had no contact with animals, but her house bordered on bushland.
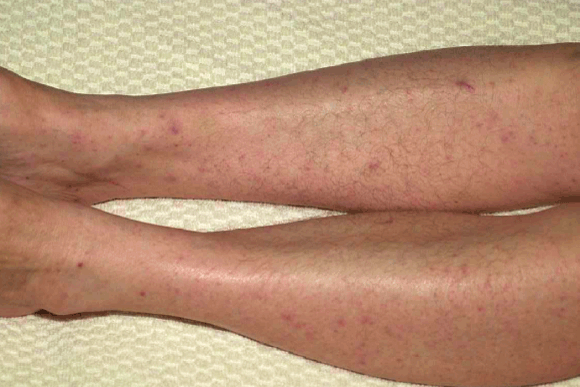
On admission she appeared unwell, with tachycardia and temperature over 39°C. There was a diffuse maculopapular rash (Box 2), but no eschar, and crackles were audible in the left lung base. Chest x-ray showed bilateral reticular infiltrates that were most pronounced in the left mid and lower zones. Results of laboratory tests, including rickettsial serological testing, culture and polymerase chain reaction (PCR), are shown in Box 3.
There was no initial response to empiric therapy with flucloxacillin, gentamicin and azithromycin. Three days after admission, she was reviewed by an infectious diseases physician and her antibiotic therapy was changed to 100 mg doxycycline twice daily, which resulted in rapid improvement of her condition, with resolution of fever and near-complete disappearance of rash within 48 hours. Doxycycline therapy was continued for 7 days. A week after discharge, she was well and there were no clinical or laboratory abnormalities.
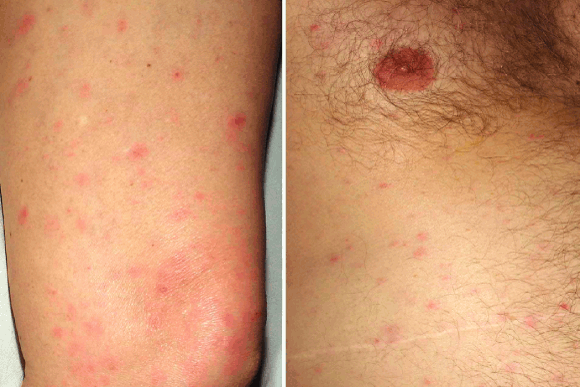
In December 2002, a 74-year-old man from the south coast of SA (Box 1) was referred after 5 days of high fever, rigors, malaise, and severe prostration with mild confusion. He had a generalised, non-pruritic maculopapular rash, predominantly on the trunk but also on the extremities, including the palms and soles (Box 2). There was no history of recent travel or animal or arthropod exposure, but his house was on the edge of bushland. He had noticed numerous lizards in the vicinity of his house, but had not had any direct contact.
On examination, he appeared unwell, with a temperature of 40°C and diffuse rash, but no other abnormal physical findings. Empiric therapy with flucloxacillin, ceftriaxone and gentamicin was started, but doxycycline (100 mg twice daily) was substituted 24 hours later after review by an infectious diseases physician. Within 48 hours, the fever resolved and he was discharged home. On review 6 weeks later, he was well, with no abnormal clinical or laboratory findings.
In March 2003, a 44-year-old woman from a southern Adelaide suburb (Box 1) presented after 8 days of lethargy, fever, rigors, headache, arthralgia and muscle pains; 4 days after the initial symptoms appeared, a maculopapular rash appeared over her trunk and limbs. She gave a history of probable insect bite 4 or 5 days before symptom onset.
She had a temperature of 39°C and a widespread macular, blanching eruption over her trunk and limbs, including the palms and soles. A number of blood cultures, as well as serological tests for cytomegalovirus, Epstein–Barr virus, parvovirus B19, Ross River and Barmah Forest viruses, and also leptospirosis, were negative. On Day 4 of admission (12 days after onset of symptoms), she started a 7-day course of doxycycline (100 mg twice daily), and the fever resolved within 24 hours. At follow-up 3 weeks later, there was complete resolution of rash and normalisation of all haematological and biochemical parameters.
In May 2003, a 58-year-old man from a southern Adelaide suburb (Box 1) presented with 7 days of high fever, followed 2 days later by a macular rash on the trunk and limbs, which resolved within 72 hours. On the night of admission he awoke with dyspnoea, but there was no cough or sputum. There was no history of tick bite or recent direct animal exposure.
On examination, his temperature was 38.4°C and there were bilateral basal chest crackles. There was no rash or eschar. Chest x-ray showed bilateral basal interstitial shadowing. After initial treatment with intravenous benzylpenicillin and erythromycin, his antibiotic therapy was changed to 100 mg oral doxycycline twice daily as treatment for presumed atypical pneumonia. Acute and 2-week convalescent serological tests showed no evidence of recent infection with Chlamydophila species, legionella, Mycoplasma pneumoniae, Q fever, parvovirus B19, Epstein–Barr virus, cytomegalovirus or Rickettsia spp. PCR testing of a throat swab for M. pneumoniae and Chlamydophila spp. was negative. The fever resolved within 48 hours of starting doxycycline therapy, and he was discharged home. The patient subsequently moved interstate and was lost to follow-up.
We describe four cases of rickettsial spotted fever in the south-eastern coastal region of South Australia, where this infection has not previously been recognised. In Patients 1 and 2, there was clearcut serological evidence of acute SFG rickettsial infection, with at least a fourfold rise in antibody titre. The causal pathogen was identified in Patient 2 as R. honei. Patient 3 had a compatible illness and history of antecedent arthropod bite, and developed low level SFG rickettsial antibodies 33 days after symptom onset. Patient 4 had positive rickettsial blood culture, from which R. honei DNA was identified. In this patient there were no detectable rickettsial SFG antibodies in serum taken during the acute phase of the illness or in Week 2 of convalescence. The serological response to some SFG rickettsial infections may be delayed or even absent, especially in patients treated with tetracyclines.7
After inoculation and dissemination through the bloodstream, SFG rickettsiae proliferate within vascular endothelial cells, resulting in vasculitis and (usually) a characteristic rash. The four patients described presented with fever, maculopapular rash, severe malaise, muscle pains, and, in two cases, respiratory symptoms and signs, together with pulmonary infiltrates. In the original case series of Flinders Island spotted fever, common symptoms included fever, headache, myalgia, arthralgia, cough, and maculopapular rash.4 All our patients required hospital admission, but it is likely that mild or asymptomatic infection occurs in others and remains undiagnosed.8 In all cases, there were significant abnormal laboratory findings, including deranged liver function, activated neutrophils on blood film, and markedly elevated C-reactive protein levels (Box 3). White blood cell counts were either low or normal, and one patient had significant thrombocytopenia.
Antibiotic therapy with tetracyclines reduces the duration and severity of illness in most reported series of SFG rickettsial infection, including Flinders Island spotted fever.4,9 Symptoms and abnormalities in laboratory findings resolved rapidly for all our patients after doxycycline therapy was initiated; three of the four had had no discernible response to previous antimicrobial regimens, including, in 2 cases, macrolide drugs, which have been proposed elsewhere as a suitable alternative treatment for rickettsial SFG infections if tetracyclines are contraindicated.10
Of the four rickettsial species implicated in human infections in Australia, R. australis and R. honei cause spotted fever, which, until now, has been reported only along the eastern seaboard. Uniquely for arthropod-transmitted infections of humans, the vector of Flinders Island spotted fever has recently been shown to be Aponomma hydrosauri, a parasitic tick found on reptiles.6 This tick is found in the coastal geographic range of its host species in Tasmania, Flinders Island in Bass Strait, as well as much of southern mainland Australia, including south-eastern South Australia,11 where the cases we describe occurred. Although two of our patients gave histories suggesting arthropod bites a few days before onset of symptoms, there was no unequivocal tick exposure in any patient. This accords with the original case series of Flinders Island spotted fever, in which only four of 26 patients gave a history of tick bite, and reinforces the importance of not discounting possible tick-borne infection based on apparent lack of exposure. The interval of 2–5 days between possible tick exposure and onset of fever in two of our patients is consistent with reported incubation periods in individual cases of Flinders Island spotted fever where a tick has been isolated,4 whereas the incubation period of R. australis infection is probably somewhat longer.1 That most of our cases occurred in warmer months is also consistent with the observed seasonality of Flinders Island spotted fever,4 and with the predicted activity of A. hydrosauri.
These cases define a previously unknown focus of rickettsial spotted fever in regions of recent population growth near Adelaide, South Australia. Environmental changes bringing humans into unusual propinquity with native fauna and their arthropod ectoparasites could increase both the geographic range and number of rickettsial species causing disease. Other ecological disturbances, including climate change and complex alterations in predator–prey relationships that increase populations of biting arthropods, may also lead to increases in the incidence of arthropod-borne infection.12,13 On the other hand, our recognition of Patient 1 as a case of SFG rickettsial infection clearly resulted in heightened local awareness of this diagnosis and increased active case finding. It is possible that locally acquired cases of rickettsial infection have been presenting to clinicians for many years without being recognised. Increased awareness and improved diagnostic capability, particularly molecular-amplification and DNA-sequencing technologies, are also likely to increase the number and species range of human rickettsial diagnoses.14
The microbiology, ecology and epidemiology of SFG rickettsial infections in South Australia require further study. Meanwhile, clinicians should be aware that rickettsial infections, particularly Flinders Island spotted fever, may occur outside previously described geographic ranges in Australia. Clinically compatible cases should be further investigated with appropriate serological and microbiological tests, and empiric doxycycline therapy should be considered to shorten the duration of illness.
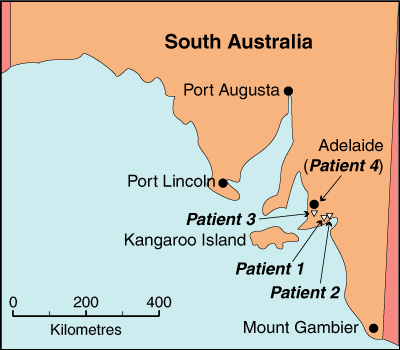
1 Map of the South Australian coast, showing the locations south of Adelaide where the four patients with rickettsial spotted fever lived
2 Diffuse maculopapular rash associated with spotted fever group rickettsial infection
Patient 1
Patient 2
3 Laboratory test results for four patients with rickettsial spotted fever
Laboratory test (reference range) |
Patient 1 |
Patient 2 |
Patient 3 |
Patient 4 |
|||||||||||
White cell count (4.0–11.0 × 109/L) |
3.3 × 109/L |
8.6 × 109/L |
8.5 × 109/L |
7.8 × 109/L |
|||||||||||
Blood film |
Left shift and toxic changes in neutrophils |
Left shift and toxic changes in neutrophils |
Left shift and toxic changes in neutrophils |
Mild reactive lymphocytosis |
|||||||||||
Platelet count (150–450 × 109/L) |
74 × 109/L |
189 × 109/L |
Normal |
194 × 109/L |
|||||||||||
Alanine aminotransferase (< 50 U/L) |
311 |
54 |
305 |
120 |
|||||||||||
C-reactive protein (< 6 mg/L) |
262 |
149 |
243 |
148 |
|||||||||||
Acute SFG antibody titre |
< 128 (Day 6)* |
< 128 (Day 6)* |
< 128 (Day 9)* |
< 128 (Day 7)* |
|||||||||||
Convalescent SFG antibody titre |
512 (Day 19)* |
> 1024 (Day 17)* |
128 (Day 33)* |
< 128 (Day 18)* |
|||||||||||
Rickettsia SFG culture of buffy coat cells and skin |
Buffy coat and skin negative (Day 9)† |
Buffy coat and skin negative (Day 8)† |
Buffy coat and skin negative (Day 12)† |
Buffy coat positive (Day 12);†no skin biopsy taken |
|||||||||||
Rickettsia SFG 17 kDa gene PCR of DNA extract from tissue |
Buffy coat and skin negative |
Buffy coat negative; skin positive |
Buffy coat negative |
Buffy coat and skin negative |
|||||||||||
17 kDa gene sequence analysis |
nd |
Ricksettia honei (100% homology) |
nd |
Ricksettia honei (100% homology) |
|||||||||||
All test samples were obtained on the day of admission to hospital unless otherwise specified: * day after symptom onset; †day of collection after symptom onset. All skin biopsies were obtained from the anterior abdominal wall. SFG = Spotted fever group. PCR = Polymerase chain reaction. nd = Not determined. |
|||||||||||||||
- 1. Sexton DJ, Dwyer B, Kemp R, Graves S. Spotted fever group rickettsial infections in Australia. Rev Infect Dis 1991; 13: 876-886.
- 2. Russell RC. Vectors vs. humans in Australia — who is on top down under? An update on vector-borne disease and research on vectors in Australia. J Vector Ecol 1998; 23: 1-46.
- 3. Dwyer BW, Graves SR, McDonald MI, et al. Spotted fever in East Gippsland: a previously unrecognised focus of rickettsial infection. Med J Aust 1991; 154: 121-125.
- 4. Stewart RS. Flinders Island spotted fever: a newly recognised endemic focus of tick typhus in Bass Strait. Part 1. Clinical and epidemiological features. Med J Aust 1991; 154: 94-99.
- 5. Chin RH, Jennens ID. Rickettsial spotted fever in Tasmania [letter]. Med J Aust 1995; 162: 669.
- 6. Stenos J, Graves S, Popov VL, Walker DH. Aponomma hydrosauri, the reptile-associated tick reservoir of Rickettsia honei on Flinders Island, Australia. Am J Trop Med Hyg 2003; 69: 314-317.
- 7. Fournier P, Jensenius M, Laferl H, et al. Kinetics of antibody responses in Rickettsia africae and Rickettsia conorii infections. Clin Diagn Lab Immunol 2002; 9: 324-328.
- 8. Graves SR, Dwyer BW, McColl D, McDade JE. Flinders Island spotted fever: a newly recognised endemic focus of tick typhus in Bass Strait. Part 2. Serological investigations. Med J Aust 1991; 154: 99-104.
- 9. Watt G, Parola P. Scrub typhus and tropical rickettsioses. Curr Opin Infect Dis 2003; 16: 429-436.
- 10. Cascio A, Colomba C, Di Rosa D, et al. Efficacy and safety of clarithromycin as treatment for Mediterranean Spotted Fever in children: a randomized controlled trial. Clin Infect Dis 2001; 33: 409-411.
- 11. Bull CM, Sharrad RD, Petney TN. Parapetric boundaries between Australian reptile ticks. Proc Ecol Soc Aust 1981; 11: 95-107.
- 12. McMichael AJ. Global climate change: will it affect vector-borne infectious diseases? Intern Med J 2003; 33: 554-555.
- 13. LoGiudice K, Ostfeld RS, Schmidt KA, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc Natl Acad Sci USA 2003; 100: 567-571.
- 14. Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev 1997; 10: 694-719.





None identified.