Clinical records
Patient 1 In 2004, a 63-year-old woman presented to the emergency department with a 1-month history of nausea and intermittent vomiting. Three weeks before presentation she was empirically prescribed esomeprazole for management of dyspepsia. A week before presentation, she stopped taking this medication, as she suspected it was exacerbating the malaise, nausea and vomiting. On presentation, she was pale, with blood pressure of 160/60 mmHg and no signs of fluid overload. Dipstick urinalysis showed blood +, protein ++, and leukocytes. Serum creatinine level was 1878 μmol/L (reference range [RR], 60–125 μmol/L), and serum urea level, 42 mmol/L (RR, 2.5–6.5 mmol/L). A normochromic, normocytic anaemia was present (haemoglobin level, 87 g/L; RR, 130–180g/L), with no eosinophilia. Urine microscopy revealed isomorphic red blood cells, some white blood cells, but no casts. Urine culture showed no growth. On admission, the patient’s remaining medications (irbesartan [150 mg daily] and atorvastatin [40 mg at night]) were also withdrawn as a precautionary measure. As acute interstitial nephritis was suspected, she was treated with methylprednisolone (500 mg daily for 3 days) followed by oral prednisone (50 mg daily). Supportive haemodialysis was begun. On Day 4 of admission, a renal biopsy was performed; results were consistent with acute interstitial nephritis. Prednisolone therapy was continued for a total of 4 weeks on a tapering dose. Supportive dialysis was required for 4 days, by which time renal function had improved. However, at follow-up 8 months later, serum creatinine level remained abnormal at 187 μmol/L (Box 1). |
Patient 2 In the same year, a 63-year-old man presented to the emergency department with a 3-week history of nausea, vomiting, weight loss and oliguria. Five weeks before presentation, he was prescribed rabeprazole by his family physician as empirical treatment for nausea and dyspepsia. This was replaced by esomeprazole a week later, as symptoms had not resolved. The patient discontinued the latter after 2 weeks’ therapy (12 days before presentation), as he had still gained no relief of symptoms. Medications on presentation were irbesartan/hydrochlorothiazide (300/12.5 mg daily), amlodipine (10 mg daily) and rofecoxib (12.5 mg daily) as required. He had been taking these medications for the previous 3 years, but used rofecoxib infrequently. On presentation, the patient was pale, with no signs of fluid retention. Dipstick urinalysis showed blood ++, protein +, and trace leukocytes. Serum creatinine and urea levels were 1110 μmol/L and 31 mmol/L, respectively. Six months previously, serum creatinine level had been 109 μmol/L. The patient also had normochromic, normocytic anaemia (haemoglobin level, 90 g/L), with no eosinophilia. Urine microscopy revealed isomorphic red blood cells, granular casts, hyaline casts, some white blood cell casts, but no red blood cell casts. Urine culture showed no growth. A clinical diagnosis was made of acute interstitial nephritis induced by a proton-pump inhibitor (PPI). The patient was admitted to hospital and treated with prednisolone (60 mg daily). Renal biopsy 2 days later confirmed acute interstitial nephritis (Box 2). Renal function improved after admission, and serum creatinine level was 600 μmol/L on discharge 5 days after presentation. Subsequently, renal function declined slowly, and, 9 months after presentation, long-term peritoneal dialysis was begun. |
In these two cases of acute interstitial nephritis, esomeprazole was implicated as the likely causative agent (although Patient 2 was also briefly exposed to rabeprazole). In both cases, renal function improved after esomeprazole was withdrawn and corticosteroid treatment begun, although Patient 2 went on to require long-term dialysis.
Omeprazole was first implicated as a cause of acute interstitial nephritis in 1992.1 Since then, 22 case reports of omeprazole-induced acute interstitial nephritis have been referenced in Medline. 2-7 Recently, pantoprazole and lansoprazole have also been implicated as causes of acute interstitial nephritis, 3,8 along with rabeprazole (unpublished data from our centre). Before the cases reported here, the Adverse Drug Reactions Advisory Committee (ADRAC) had been notified of four cases of acute interstitial nephritis induced by esomeprazole, along with six cases of acute renal failure and two of renal impairment.
In October 2004, the manufacturer of esomeprazole, AstraZeneca, reported being aware of at least 15 cases worldwide of acute interstitial nephritis possibly induced by esomeprazole, and at least 200 cases worldwide induced by omeprazole (data on file, Astra-Zeneca). Two of these esomeprazole cases and 20 of the omeprazole cases were from Australia (it is unclear whether these two esomeprazole cases were included in those notified to ADRAC). MIMS online lists acute interstitial nephritis as a complication of omeprazole but not esomeprazole.9
A recent hospital series reported drug-induced acute interstitial nephritis as the cause of biopsy-proven acute renal failure in 8% of cases, with PPIs accounting for eight of the 14 cases. 2 The diagnosis of acute interstitial nephritis is most common when renal biopsy is performed for unexplained renal impairment, with a reported prevalence in this setting of 27%.10
PPI-induced acute interstitial nephritis poses a particularly difficult diagnostic challenge, as symptoms are non-specific and may mimic the original indications for which the PPI was prescribed. Most patients diagnosed with PPI-induced acute interstitial nephritis recover renal function, but some fail to recover fully and, in extreme cases, require long-term renal replacement therapy, 2 as in Patient 2. Little is known about the relationship between the risk of PPI-induced acute interstitial nephritis and duration and dosage of PPI therapy, delay in diagnosis, or other factors. In addition, it is not clear whether the prognosis differs between acute interstitial nephritis induced by PPIs and that induced by other drugs.
Although both our patients were treated with corticosteroids, evidence for their use remains anecdotal and is not derived from randomised controlled trials. A recent report of the largest retrospective series published to date found no statistically significant difference in outcome, as determined by serum creatinine level, between patients who received corticosteroid therapy and those who did not at 1, 6 and 12 months after presentation.11
PPIs are now the third most commonly prescribed drug in Australia. Although acute interstitial nephritis is a rare complication, it is a potentially catastrophic cause of acute and chronic renal failure. All medical practitioners need to be aware of this potential class reaction. Early recognition may prevent the development of irreversible renal injury.
1 Serum creatinine level over time after presentation in Patient 1
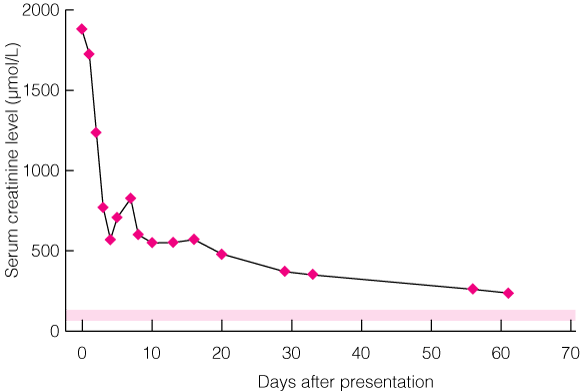
Shaded area indicates the reference range, 60–125 μmol/L.
2 Renal biopsy specimen in Patient 2
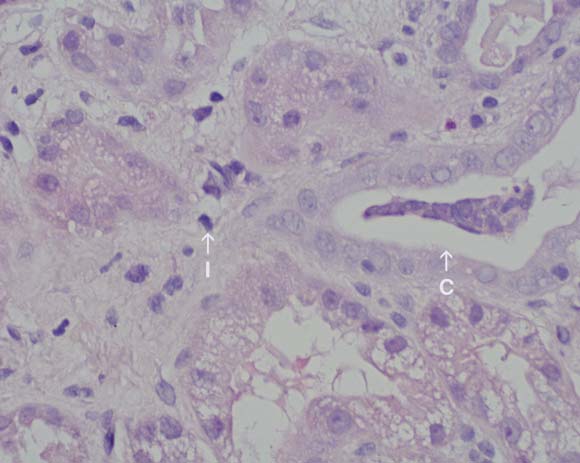
Biopsy specimen from the renal cortex taken 2 days after presentation, showing an interstitial inflammatory infiltrate composed of lymphocytes and eosinophils (I), with mild acute tubular necrosis and a cellular cast (C). (Original magnification × 40; haematoxylin–eosin stain.)
- 1. Ruffenach SJ, Siskind MS, Lien YH. Acute interstitial nephritis due to omeprazole. Am J Med 1992; 93: 472-473.
- 2. Torpey N, Barker T, Ross C. Drug induced tubulo-interstitial nephritis secondary to proton pump inhibitors: experience from a single UK renal unit. Nephrol Dial Transplant 2004; 19: 1441-1446.
- 3. Geetha D. Omeprazole-induced acute interstitial nephritis. Am J Gastroenterol 1999; 94: 3375-3376.
- 4. Post AT, Voorhorst G, Zanen AL. Reversible renal failure after treatment with omeprazole. Neth J Med 2000; 57: 58-61.
- 5. Wall CA, Gaffney EF, Mellotte GJ. Hypercalcaemia and acute interstitial nephritis associated with omeprazole therapy. Nephrol Dial Trans 2000; 15: 1450-1452.
- 6. Myers RP, McLaughlin K, Hollomby DJ. Acute interstitial nephritis due to omeprazole. Am J Gastroenterol 2001; 96: 3428-3431.
- 7. Delve P, Lau M, Yun K, Walker R. Omeprazole-induced acute interstitial nephritis. N Z Med J 2003; 116: 332.
- 8. Ra A, Tobe SW. Acute interstitial nephritis due to pantoprazole. Ann Pharmacother 2004; 38: 41-45.
- 9. MIMS online [electronic database]. Available at: www.mims.hcn.net.au (accessed Dec 2004: subscription required).
- 10. Farrington K, Levison DA, Grenwood RN, et al. Renal biopsy in patients with unexplained renal impairment and normal size kidney. Q J Med 1989; 70: 221-233.
- 11. Clarkson MR, Giblin L, O’Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004; 19: 2778-2783.





N G received a travel grant from Roche and Amgen to present this case at the annual meeting of the American Society of Nephrology. P L C received support from Amgen to attend a meeting of the Australian and New Zealand Society of Nephrology and speaker fees from Bristol–Myers Squibb.