The radiopharmaceutical fluorine-18-fluorodeoxyglucose (FDG), an analogue of glucose, has a high rate of uptake in a wide range of tumours. Positron emission tomography (PET) employing FDG-PET is an accurate functional imaging technique in the diagnosis, staging, restaging and therapeutic monitoring of many common cancers.1 In addition, PET scanning often leads to appropriate alterations in patient management,2 and its use has been associated with improved survival among patients with lung cancer, probably because of improved treatment selection and delivery.3 Recently, Medicare funding for FDG-PET has been allocated for a limited period for the purpose of collecting more data to document the clinical efficacy of the procedure in the Australian context. Although restricted to specific indications and to eight PET machines, this federal funding does provide greater public access and clinical exposure to the technique.
Despite the proven benefits of FDG-PET, imaging is a lengthy process. One reason for this is that accurate determination of FDG uptake in tissue requires correction for the effects of attenuation. With traditional PET scanners, attenuation is routinely measured using radioactive sources in a process which increases the duration of the scan by about 30%. Furthermore, as PET images contain limited anatomical information, physiological uptake into the heart, skeletal muscle and gut can cause diagnostic dilemmas. Increased uptake in inflammatory and other non-malignant but diseased tissues can also cause confusion.4,5 Thus, PET has traditionally been used to complement structural imaging, which is required for accurate T staging and to maximise diagnostic accuracy.
Recently, scanners incorporating PET and computed tomography (CT) have become available, allowing sequential CT and PET imaging with the same device. The CT produces an attenuation map that surpasses the quality produced from conventional radioactive sources, and in a smaller fraction of the time. The CT images can also be efficiently co-registered with the PET data so that the “fusion images” provide a structural template for simultaneous correlation with the metabolic image. That this innovation promises to become the most comprehensive diagnostic tool in oncological imaging is shown by studies which have shown that these combined PET/CT scanners improve the accuracy of cancer evaluation compared with either device used alone.6 Already, 65% of current PET scanner sales in the United States are PET/CT devices7 and these machines are expected to dominate the market in coming years.
Four of the 12 PET scanners now operating in Australia are PET/ CT devices, and upgrades are planned for several other standalone PET scanners. The first Australian PET/CT scanner was commissioned in January 2002 at the Peter MacCallum Cancer Centre (PMCC), Melbourne, partly funded through a Commonwealth capital grant. Having now performed more than 5500 scans, we felt it opportune to describe our experience with this major innovation in diagnostic imaging.
The PET/CT scanner at PMCC (Discovery LS; General Electric Medical Systems) comprises a 4-slice multidetector helical CT scanner (Lightspeed Plus) and a dedicated PET scanner using BGO crystals (Advance Nxi).
Patients are prepared for the PET/CT scans in the usual fashion for PET scanning. The CT scan is done before the PET scan. For oncological indications, an extended body survey is usually acquired, typically including images from the skull base to the proximal thighs. The typical acquisition time is less than a minute for the CT scan. Although the CT device has high-level diagnostic capability, the CT component in our protocol is performed as a non-contrast, low-radiation-dose scan. It is performed primarily for attenuation correction and anatomical co-registration. The CT scan is not considered to be of full diagnostic quality and is not separately reported. Next, without the patient changing position, a PET scan encompassing the same imaging field is performed. The combined PET/CT scan takes about 30 minutes, which is roughly two-thirds of the time required for a PET scan performed on a standalone device. The estimated radiation dose for the PET and the low-dose CT scan on our scanner is about 7 mSv each, giving a combined PET/CT scan radiation dose of 14 mSv, which is consistent with a previous report8 and similar to the dose for a typical whole-body diagnostic CT scan.
The resulting PET/CT studies are interpreted at a computer workstation with dedicated software allowing review of attenuation-corrected PET, CT and fusion PET/CT images in three orthogonal planes (Box 1). Both the CT and PET/CT fusion images are used to localise PET uptake abnormalities.
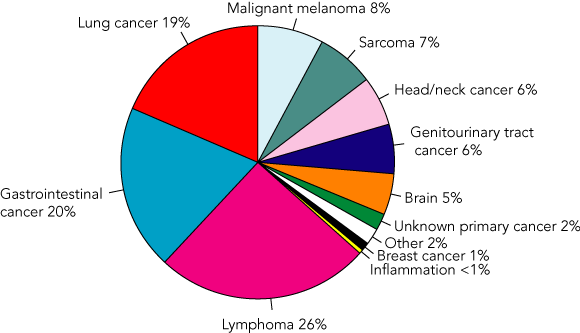
PMCC is a quaternary institution and Australia’s only standalone dedicated cancer-care centre. PET scanning was introduced at PMCC in September 1996 and one PET/CT and one standalone PET scanner are currently in clinical use. Registered Peter MacCallum patients, as well as patients referred from external sources specifically for PET, are scanned. However, Medicare reimbursement is available only to patients scanned on the PET/CT scanner, and only for a limited range of clinical indications. The casemix of studies performed on the PET/CT scanner is illustrated in Box 2, but it should be recognised that this is greatly influenced by funding constraints. Oncological indications account for 95% of the PET/CT scans performed, and the major tumour groups scanned included lymphoma (Box 3), gastrointestinal (Box 4) and lung malignancies.
Current Medicare funding arrangements do not extend to all indications where PET is clinically useful. To analyse the contribution of Medicare funding for our PET workload, we examined the subset of our patients who were scanned in 2003, at a time when referral patterns had stabilised. Of the 3338 PET scans performed, 2470 were performed on the PET/CT scanner and 1728 (70% of 2470) qualified on clinical indication and billing status for Medicare reimbursement. However, 52% (1728/3338) of the total PET caseload was funded through Medicare. Funding for studies not eligible for Medicare reimbursement included direct patient billing, research grants and PMCC consolidated revenue.
The Medicare scheduled fee for a PET scan is currently about $950. The unit cost of a PET/CT scan is highly dependent on actual equipment used, radioisotope cost, patient throughput and staffing. However, the cost of PET has decreased with falling equipment and isotope prices, more efficient service delivery models, and effective equipment use.9 Regrettably, Medicare reimbursement is currently not available for diagnostic CT scans performed on a PET/CT scanner, and this restriction limits the efficiency of capital utilisation in some settings.
CT-based attenuation correction can be achieved in a fraction of the time required for radioactive sources10 and with comparable or superior statistical quality. By decreasing total scan time by more than 30%, patient comfort and convenience is significantly improved. Despite the higher purchase and maintenance costs of PET/CT, the large increase in patient throughput capacity allows substantial amortisation of equipment, staff and infrastructure costs. Because of the short physical half-life of 18F, higher throughput also decreases daily radiopharmaceutical requirements, potentially further reducing the costs of PET. These efficiencies, combined with the rapidly declining cost of equipment, will allow PET/CT imaging to be performed for little additional cost, reinforcing the cost benefits which have been demonstrated for PET alone.11
One of the major difficulties in interpreting FDG-PET scans is uptake into physiological structures, particularly muscle and the gastrointestinal tract.4,5 With the advent of PET/CT, brown fat activity (see Box 5) can be easily differentiated from malignant disease, eliminating a significant interpretive problem.12,13 Differentiation of pathological and physiological uptake of FDG is particularly problematic in regions of complex anatomy such as the neck, abdomen and pelvis. In the neck, FDG uptake in muscular structures, lymphoid aggregations, thyroid gland and brown fat can mask or mimic nodal metastases. Similarly, in the epigastrium, physiological uptake in the lower oesophageal sphincter, stomach, pylorus and transverse colon related to smooth muscle activity, as well as in the diaphragmatic crura, can make interpretation difficult. Categorising physiological uptake often becomes more difficult after treatments that distort normal anatomy. Co-registration of the CT and PET images enables physiological uptake to be more confidently assigned to normal structures, and pathological uptake to be both recognised and localised.14
Improved image visualisation has been an important focus of software development for many of the major global instrumentation manufacturers, and these developments can readily be applied to PET/CT. Providing a precise anatomical address for the functional imaging signature in the form of fused images has great aesthetic appeal, and such images are readily understood by physicians who do not have specific expertise in imaging. This improves the transfer of diagnostic information and has probably been a large contributing factor to the rapid clinical acceptance of this technology.15
Attempts at histological characterisation can be problematic in patients with cancer. The availability of anatomical information from PET/CT can assist in selecting the most accessible mass for biopsy. After treatment, residual masses may not contain any active tumour, and the presence of residual uptake can guide which masses require biopsy. In addition, tissue heterogeneity is a common feature within individual malignant masses, particularly in sarcomas, where high-grade tumour can co-exist with acellular matrix components and low-grade tumour elements. Because of the high uptake of FDG in aggressive tumours, intermediate FDG uptake in low-grade tumours and very low FDG uptake in necrotic or fibrotic tissue, PET/CT imaging can be used to guide biopsy to the most metabolically active regions of structural masses (Box 6). This has two potential advantages: the likelihood of non-diagnostic biopsy may be reduced,16 and the biopsy will more likely reflect the most biologically active, and therefore prognostically significant, tumour elements.17,18
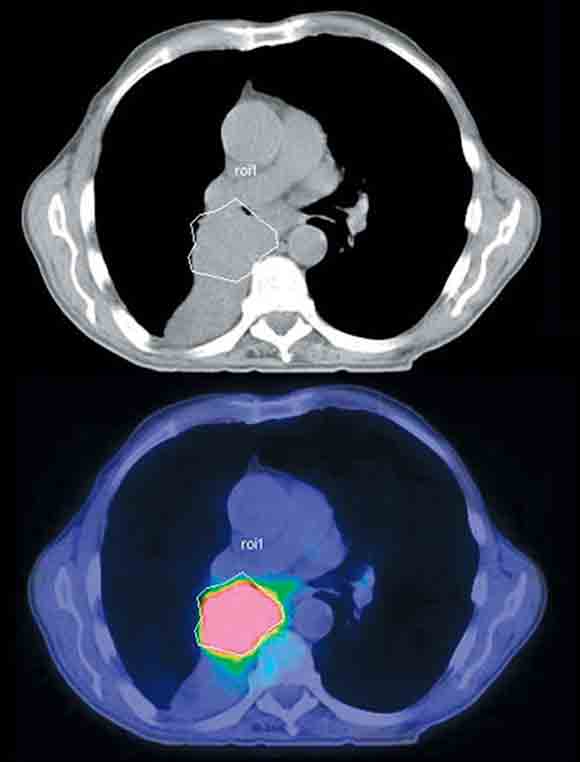
Improved anatomical localisation provided by PET/CT translates directly to improved treatment planning. Loco-regionally confined cancers are generally treated with greatest success by aggressive local therapies, particularly surgery. In cases unsuitable for surgery, radical radiotherapy is increasingly being combined with concurrent chemotherapy. These local therapies can only be effective if all of the tumour is contained within the treatment volume.19 The greater sensitivity and specificity of PET for determining the extent of active malignancy is complemented by the superior ability of PET/CT to determine the true stage of disease, thereby improving the clinician’s ability to select patients suitable for aggressive local therapy. In addition, targeting of radiation therapy can also be improved by determining the gross tumour volume by PET (Box 7). Preliminary studies have suggested that PET/CT is ideal for this purpose.20
It is clear that respiratory movement can lead to misregistration of PET and CT lesions, especially common in the lower thorax (Box 8) and upper abdomen. This does not usually pose a diagnostic problem as long as it is recognised.21
Data acquisition for reconstructing PET images occurs over several minutes and therefore integrates the effect of respiratory movement. This increases the apparent superoinferior size of lesions near the base of the lungs that move primarily in the coronal plane during respiration and the anteroposterior dimensions of lesions in the anterior aspect of the lungs that move mainly in the axial plane.
CT scanning is, however, very rapid, and multislice scanners can acquire images sufficiently fast to effectively “freeze” respiratory motion. Alternatively, CT images can be acquired during breath-holding at a given phase of respiration.
When CT is used for attenuation correction, it is necessary to extrapolate from the attenuation characteristics obtained using relatively low-energy x-rays to those determined for 511-keV annihilation photons of PET. This process can introduce artefacts in relationship to dense objects such as metallic implants and barium.22,23 Such artefacts are easy to recognise, and reconstruction algorithms are being refined to eliminate their occurrence. Non-attenuation-corrected images should be reviewed when high accumulation of FDG is observed in relationship to radiologically dense objects.
The great majority of clinical applications for our combined PET/CT scanner are for oncological indications, with the actual casemix heavily influenced by the availability of Medicare reimbursement.
Consistent with the published literature, we have found that PET/CT improves diagnostic certainty, especially in areas of dense anatomy such as head and neck and abdomen and pelvis, and aids in treatment planning, particularly for radiotherapy.24,25 However, the major advantages of PET/CT over PET and CT alone are logistical. Given the arduous management schedules which confront many patients with cancer, logistical advantages represent a major advance. Increasingly, our referring clinicians are requesting PET/CT as the first imaging tool, as the information provided is considered vital in management decision-making. Thus, PET/CT represents an important evolution in medical imaging, combining both anatomical and functional information in a single study.26 The transition from PET and CT to PET/CT appears to be inevitable as the medical and oncological communities become more familiar with the new technology, but the pace of change will be heavily influenced by restrictions imposed by Medicare reimbursement policy.
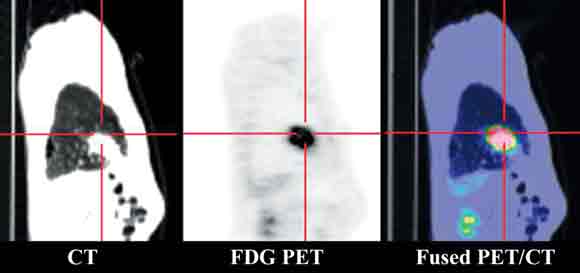
1 Typical positron emission tomography/computed tomography (PET/CT) workstation display
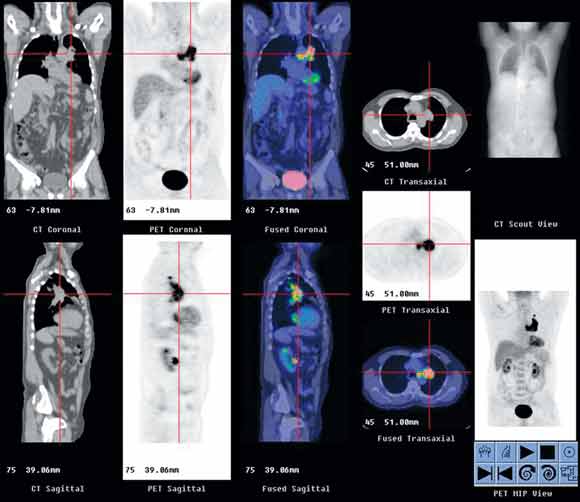
In this patient with cancer of the left lung, the display shows PET (black-and-white images), CT and PET/CT fusion (colour images) in three orthogonal planes, as well as CT scout image and whole-body PET images in AVI (audio-video-interleaved) format.
3 Positron emission tomography/computed tomography images of Hodgkin’s disease staging
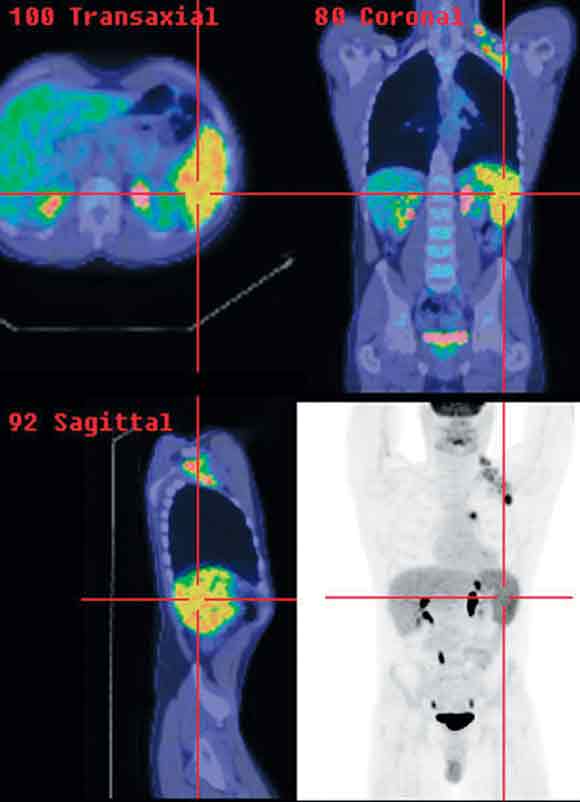
Clinical stage I left axillary disease was upstaged to stage IIIs because of mediastinal nodal and splenic involvement detected on PET/CT.
4 Positron emission tomography/computed tomography images of oesophageal cancer staging
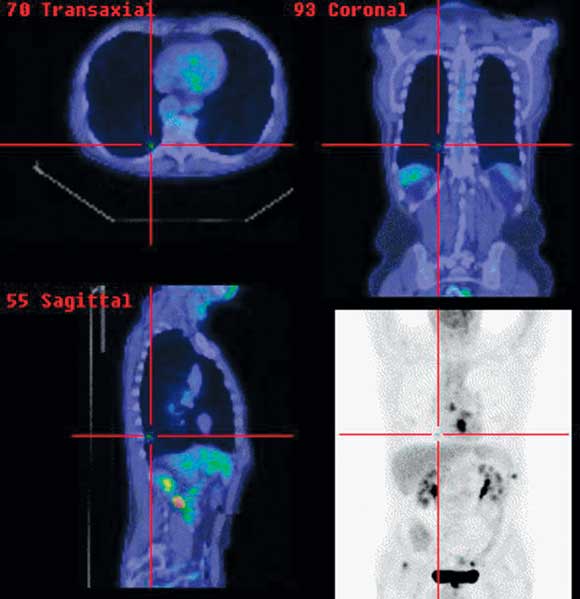
Primary distal oesophageal tumour, mediastinal nodal disease and solitary pulmonary metastasis (cross-hairs).
5 Positron emission tomography/computed tomography images of brown fat uptake in a young woman
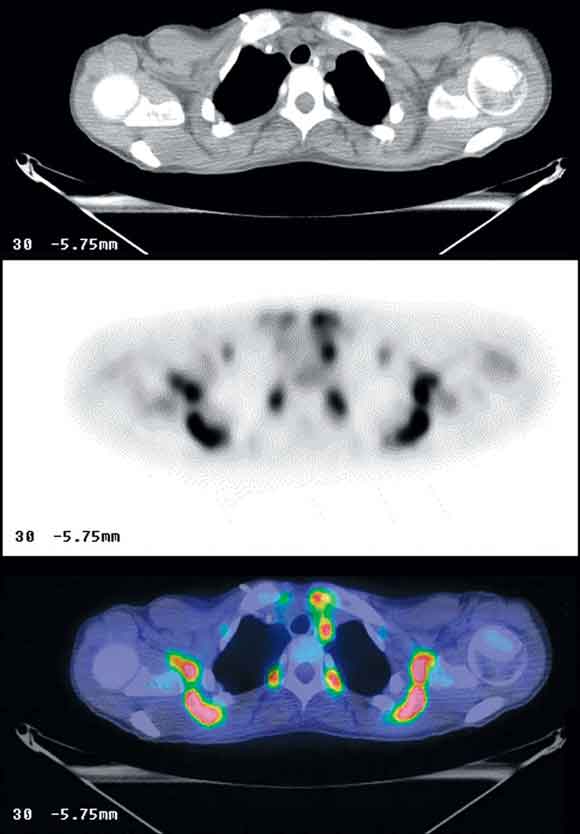
Shoulder girdles, superior mediastinum and paravertebral regions are shown in axial computed tomography (upper image), fluorine- 18-fluorodeoxyglucose positron emission tomography (middle image) and fused PET/CT (bottom image) slices, demonstrating high metabolic activity in the low density fatty tissue.
6 Positron emission tomography/computed tomography guided biopsy of a left scapular soft tissue mass
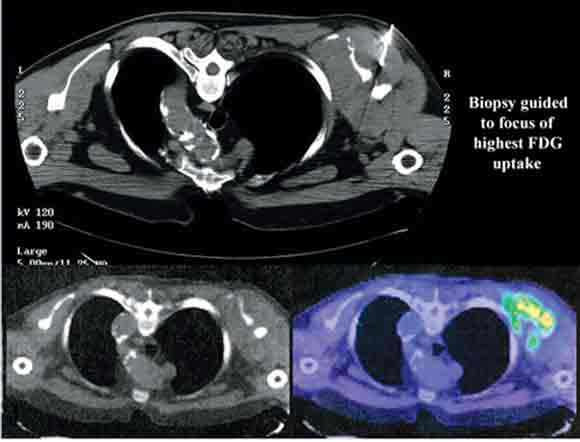
Such images allow representative histological sampling of the most metabolically active region within a heterogeneous mass lesion.
- W F Eddie Lau1
- David S Binns2
- Robert E Ware3
- Shakher Ramdave4
- Alexander G Pitman5
- Rodney J Hicks6
- Peter MacCallum Cancer Centre, Melbourne, VIC.
None identified.
- 1. Bomanji JB, Costa DC, Ell PJ. Clinical role of positron emission tomography in oncology. Lancet Oncology 2001; 3: 157-164.
- 2. Flamen P, Stroobants S, Van Cutsem E, et al. Additional value of whole body positron emission tomography with fluorine-18-2-fluoro-2-deoxy-D-glucose in recurrent colorectal cancer. J Clin Oncol 1999; 17: 894-901.
- 3. MacManus MR, Hicks R, Fisher R, et al. FDG-PET-detected extracranial metastasis in patients with non-small cell lung cancer undergoing staging for surgery or radical radiotherapy — survival correlates with metastatic disease burden. Acta Oncol 2003; 42: 48-54.
- 4. Shreve PD, Anzai Y, Wahl RL. Pitfalls in oncologic diagnosis with FDG PET imaging; physiologic and benign variants. Radiology 1999; 19: 61-77.
- 5. Gary JR, Wegner EA, Fogelman I. Pitfalls and artifacts in FDG PET and PET/CT oncologic imaging. Semin Nucl Med 2004; 34: 122-133.
- 6. Schoder H, Larson SM, Yeung WD. PET/CT in oncology: integration into clinical management of lymphoma, melanoma and gastrointestinal malignancies. J Nucl Med 2004; 45: 72S-81S.
- 7. Czermin J, Schelbert H. PET/CT Imaging: facts, opinions, hopes, and questions. J Nucl Med 2004; 45: 1S-3S.
- 8. Wu TH, Huang YH, Lee JJS, et al. Radiation exposure during transmission measurements: comparison between CT and germanium-based techniques with a current PET scanner. Eur J Nucl Med Mol Imaging 2004; 31: 38-42.
- 9. MacManus MM, Peters L, Duchesne G, et al. How should we introduce clinical PET in UK? Oncologists need to have a clearer view [editorial]. Clin Oncol 2004; 16: 492-493.
- 10. Kamel E, Hany TF, Burger C, et al. CT vs 68Ge attenuation correction in a combined PET/CT system: evaluation of the effect of lowering the CT tube current. Eur J Nucl Med 2002; 29: 346-350.
- 11. Keith CJ, Miles KA, Griffiths MR, et al. Solitary pulmonary nodules: accuracy and cost-effectiveness of sodium iodide FDG-PET using Australian data. Eur J Nucl Med Mol Imaging 2002; 29: 1016-1023.
- 12. Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in supraclavicular area (“USA-fat”): description on 18F-FDG PET/CT. J Nucl Med 2003; 44: 170-176.
- 13. Yeung HWD, Grewal RK, Gonen M, et al. Patterns of F18 FDG uptake in adipose tissue and muscle: a potential source of false-positives for PET. J Nucl Med 2003; 44: 1789-1796.
- 14. Bar-Shalom R, Yefremov N, Guralnik L, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 2003; 44: 1200-1209.
- 15. Bradley JD, Perez CA, Dehdashti F, Siegel BA. Implementing biologic target volumes in radiation treatment planning for non-small cell lung cancer. J Nucl Med 2004; 45: 96S-101S.
- 16. Beggs AD, Hain SF, Curran KM, O’Doherty MJ. FDG-PET as a “metabolic biopsy” tool in non-lung lesions with indeterminate biopsy. Eur J Nucl Med 2002; 29: 542-546.
- 17. Hain SF, O’Doherty MJ, Bingham J, et al. Can FDG PET be used to successfully direct preoperative biopsy of soft tissue tumours? Nucl Med Commun 2004; 24: 1139-1143.
- 18. Di Chiro G. PET using FDG in brain tumours. A powerful diagnostic and prognostic tool. Invest Radiol 1987; 22(5): 360-371.
- 19. MacManus MP, Hicks RJ, Matthews JP, et al. High rate of detection of unsuspected distant metastases in apparent-stage III non-small cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys 2001; 50: 287-293.
- 20. Ciernick IF, Dizendorf E, Baumert BG, et al. Radiation treatment planning with an integrated positron emission and computer tomography (PET/CT): a feasibility study. Int J Radiat Oncol Biol Phys 2003; 57: 853-863.
- 21. Osman MM, Cohade C, Nakamoto Y, et al. Clinically significant inaccurate localization of lesions with PET/CT: frequency in 300 patients. J Nucl Med 2003; 44: 240-243.
- 22. Goerres GW, Hany TF, Kamel E, et al. Head and neck imaging with PET and PET/CT: artefacts from dental metallic implants. Eur J Nucl Med 2002; 29: 367-370.
- 23. Cohade C, Osman M, Nakamoto Y, et al. Initial experience with oral contrast in PET/CT: phantom and clinical studies. J Nucl Med 2003; 44: 412-416.
- 24. Wahl RL. Why nearly all PET of abdominal and pelvic cancers will be performed as PET/CT. J Nucl Med 2004; 45: 82S-95S.
- 25. Goerres GW, Von Schulthess GK, Steinert HC. Why most PET of lung and head-and-neck cancer will be PET/CT. J Nucl Med 2004; 45: 66S-71S.
- 26. Townsend DW, Carney JPJ, Yap JT, Hall NC. PET/CT today and tomorrow. J Nucl Med 2004; 45: 4S-14S.





Abstract
Metabolic imaging with fluorine-18-fluorodeoxyglucose positron emission tomography (FDG-PET) is increasing rapidly worldwide because of superior accuracy compared with conventional non-invasive techniques used for evaluating cancer.
Limited anatomical information from FDG-PET images alone dictates that complementary use with structural imaging is required to optimise benefit.
Recently, combined positron emission tomography/computed tomography (PET/CT) scanners have overtaken standalone PET scanners as the most commonly purchased PET devices.
We describe our experience of over 5500 scans performed since the first PET/CT scanner in Australia was commissioned at the Peter MacCallum Cancer Centre (PMCC), Melbourne, in January 2002.
Clinical indications for PET/CT scans performed at PMCC largely reflect current Medicare reimbursement policy.
Advantages of PET/CT include greater patient comfort and higher throughput, greater diagnostic certainty and accuracy, improved biopsy methods, and better treatment planning.
We believe PET/CT will underpin more effective and efficient imaging paradigms for many common tumours, and lead to a decrease in imaging costs.