Two patients with anorexia nervosa and raised serum creatinine levels were found to have nephrocalcinosis on renal biopsy, an association not previously described.
Clinical records
Patient 1
A 26-year-old woman was referred with a serum creatinine level of 0.21 mmol/L (reference range [RR], 0.03–0.11 mmol/L).
She had a 5-year history of anorexia nervosa, laxative misuse, and unexplained intermittent hypercalcaemia requiring hospital admissions (calcium, > 3.0 mmol/L). During one admission, she disclosed taking a vitamin preparation containing vitamin D3 200 IU and calcium phosphate 40 mg. Investigations for hypercalcaemia included assays of parathyroid hormone, parathyroid hormone-related peptide, vitamin D, 1,25-hydroxy vitamin D, osteocalcin, serum cortisol, serum angiotensin-converting enzyme, and 24-hour urinary calcium; thyroid function tests; and serum and urine protein electrophoresis. These were all in the normal range. A urine diuretic screen was negative on two occasions.
Bone mineral density measurement was normal.
On examination, the patient was thin, weighing 40 kg (height, 160 cm; body mass index, 15.6 kg/m2). Her blood pressure was 110/80 mmHg. Estimated glomerular filtration rate (GFR) was 29 mL/min per 1.73 m2 (normal mean value in young women is 125 mL/min per 1.73 m2).
Urinalysis showed 18 000 erythrocytes/mL (RR, < 10 000/mL) and 0.17 g/day of proteinuria (RR, < 0.1 g/day). Autoimmune, hepatitis B and C serology were negative. C3 was 0.68 g/L (RR, 0.81–1.66 g/L) and C4 was 0.17 g/L (RR, 0.12–0.42 g/L). Her serum electrolytes at presentation are shown in Box 1.
Ultrasound showed kidneys 9.5 cm and 9.3 cm in length, with possible calcification of the pyramids adjacent to the upper pole calyces. There was no calcification on plain abdominal x-ray. Her renal biopsy showed calcification in degenerate tubular basement membranes within the interstitium, and in three of 36 glomeruli. There was widespread interstitial fibrosis indicating chronic damage. One glomerulus showed basement membrane reduplication, but immunoperoxidase staining was negative.
Eighteen months later, her renal function has remained unchanged.
Patient 2
A 31-year-old woman was referred by her general practitioner because of a rise in serum creatinine level from 0.16 to 0.41 mmol/L.
An eating disorder had been diagnosed 8 years earlier. Two years previously, diarrhoea and abdominal pain were investigated. A malabsorption screen, upper endoscopy, and small bowel biopsy were all normal. Colonoscopy showed melanosis coli, consistent with laxative misuse.
One year before the current admission, she had been hospitalised with hypokalaemia and renal failure (Box 1). Her potassium was corrected with intravenous replacement. She discharged herself the following day.
She reported having 30 bowel actions a day, and was taking spironolactone 25 mg daily, effervescent potassium chloride (14 mmol K+) 6 tablets/day, diazepam 2 mg as required, and diclofenac 50 mg as required. Her blood pressure was 100/60 mmHg, and she weighed 43.6 kg (height, 162.5 cm; body mass index, 16.5 kg/m2).
Electrolytes on admission are shown in Box 1. Her serum calcium levels were high on two occasions, but normal on others. Mid-stream urine had no erythrocytes or pyuria, and 24-hour urine protein was 0.44 g/day. Ultrasound showed echogenic kidneys without calcification. Serum complement and autoimmune serology were negative. Twenty-four-hour urine biochemistry showed potassium 17 mmol/day (RR, 25–100 mmol/day); sodium 21.0 mmol/day (RR, 40–210 mmol/day), calcium 0.6 mmol/day (RR, 2.5–7.5 mmol/day); oxalate 0.23 mmol/day (RR, 0.04–0.34 mmol/day); and phosphate 18.0 mmol/day (RR, 10–40 mmol/day). Bone mineral density measurement was normal. Her creatinine level reduced to 0.23 mmol/L (estimated GFR, 26 mL/min per 1.73 m2) before she underwent a renal biopsy (Box 2).
She subsequently had multiple episodes of hypokalaemia (potassium 2.0 mmol/L) despite large quantities of potassium supplements being supplied, with a positive urine laxative screen on two occasions. Serum calcium was intermittently high, the highest being 3.0 mmol/L (corrected for albumin).
One year after her renal biopsy, nasogastric feeding increased her weight from 30 kg to 38 kg after 5 weeks.
A year later, she developed pneumonia and septic shock, and died.
A key clinical feature of anorexia nervosa is “the relentless pursuit of thinness”1 and a “desperate need to grow thinner”.2 People with this disorder often engage in behaviour that affects fluid and electrolyte balance, including vomiting, laxative or diuretic misuse, fluid restriction, and the injudicious use of health supplements.
Anorexia nervosa can cause significant renal problems, some of which may have contributed to the development of nephrocalcinosis in our patients. Electrolyte disturbances include hypokalaemia, hyponatraemia, hypercalcaemia, hypomagnesaemia and hypophosphataemia.3,4 Hypokalaemia with metabolic alkalosis indicates either vomiting or diuretic misuse, whereas metabolic acidosis indicates laxative misuse.4 Hypokalaemia is rare in the absence of these behaviours.5 Body phosphate stores can be depleted, and refeeding can exacerbate hypophosphataemia, with serious consequences such as seizures and myocardial dysfunction.6 Renal function may also be affected by volume depletion.
Nephrolithiasis has been reported in patients with anorexia nervosa,7-11 but, to our knowledge, nephrocalcinosis has not. Nephrocalcinosis results from excessive calcium deposition in the kidney, and renal failure may result from tubular cell injury, tubular obstruction by calcified debris, and atrophy of nephrons. Chronic inflammation and interstitial fibrosis accompany these changes.12 In a series of 350 patients with nephrocalcinosis, the commonest causes were primary hyperparathyroidism (34.1%), distal renal tubular acidosis (20.9%), and medullary sponge kidney (11.7%).13
Medullary calcification is typical in most of these conditions, but neither of our patients had computed tomography to demonstrate medullary calcification. Both patients had hyperphosphataemia secondary to reduced glomerular filtration rate (GFR), causing an elevated calcium-phosphate product, which predisposed them to tissue calcification. However, an alternative cause for impaired GFR was not found in either patient. Both the amount of calcification and its presence in glomerular and tubular basement membranes disclosed by renal biopsy argue against calcification secondary to other renal disease.
Hypercalcaemia is the most likely cause of the nephrocalcinosis in Patient 1, probably due to use of a vitamin D preparation. However, vitamin D was not elevated on the occasion it was measured. In Patient 2, diclofenac use may have contributed to fluctuations in renal function, but does not explain the biopsy findings.
Chronic severe hypokalaemia and laxative misuse characterised Patient 2, and in her case, diarrhoea and laxative misuse were the most prominent potential causes for hypokalaemia. However, her metabolic alkalosis is more consistent with diuretic misuse or vomiting (or both) than diarrhoea. Her low urinary potassium excretion does not disprove diuretic misuse, because she was taking a potassium-sparing diuretic (spironolactone), and her diuretic misuse may have been intermittent. Indeed, chronic use of frusemide for weight control has been associated with nephrocalcinosis in patients without anorexia nervosa.14,15 Chronic diarrhoea may contribute to nephrocalcinosis by causing volume depletion. Nephrocalcinosis associated with acute renal failure has been reported in relation to an oral sodium phosphate preparation used for bowel preparation for colonoscopy.16
Prolonged hypokalaemia can cause tubular atrophy, damage to tubular cells, interstitial lymphocyte infiltration, interstitial fibrosis and juxtaglomerular apparatus hyperplasia.17 Chronic tubulo-interstitial nephritis due to hypokalaemia has been described as a cause of end-stage renal disease in anorexia nervosa patients18 and may explain some of the chronic damage observed in Patient 2.
1 Electrolyte results for the two patients
|
Patient 1 |
Patient 2 |
Reference |
||||||||||||
|
Admission |
1 year prior |
Admission |
||||||||||||
Sodium (mmol/L) |
134 |
136 |
131 |
135–145 |
|||||||||||
Potassium (mmol/L) |
4.1 |
2.0 |
2.6 |
3.5–5.0 |
|||||||||||
Chloride (mmol/L) |
100 |
76 |
77 |
95–107 |
|||||||||||
Bicarbonate (mmol/L) |
25 |
41 |
36 |
23–31 |
|||||||||||
Urea (mmol/L) |
7.2 |
15.3 |
15.0 |
2.5–7.7 |
|||||||||||
Creatinine (mmol/L) |
0.214 |
0.285 |
0.410 |
0.03–0.11 |
|||||||||||
Calcium* (mmol/L) |
2.70 |
2.79 |
2.41 |
2.10–2.60 |
|||||||||||
Albumin (g/L) |
38 |
27 |
26 |
36–48 |
|||||||||||
Phosphate (mmol/L) |
2.02 |
2.07 |
3.35 |
0.60–1.40 |
|||||||||||
Ca x P product (mg2/dL2) |
5.5 |
Not measured |
8.1 |
< 5.8† |
|||||||||||
Magnesium (mmol/L) |
0.87 |
Not measured |
1.81 |
0.70–1.00 |
|||||||||||
* Calcium value has been corrected for albumin. |
|||||||||||||||
2 Renal biopsy from Patient 2
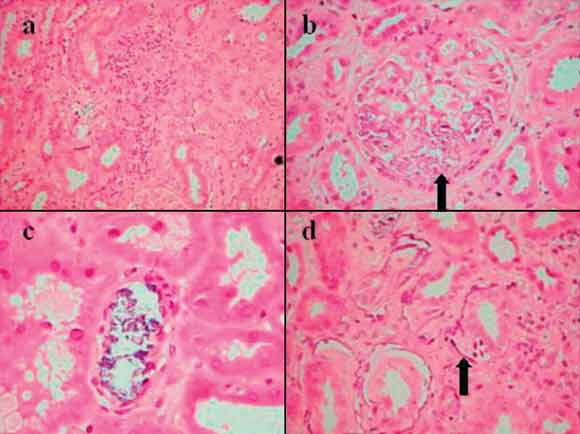
Six of 27 glomeruli were totally sclerosed and there was widespread interstitial fibrosis with tubular atrophy and interstitial lymphocytosis (a). Calcification was seen in glomerular basement membranes (b, arrow), within tubule lumens (c) and in tubular basement membranes (d, arrow). There was also intimal thickening of interlobular arteries, indicating arteriosclerosis and there were no significant abnormalities on immunofluorescence or electron microscopy. (Haematoxylin and eosin stain; original magnifications (a) × 40, (b) and (d) × 100, (c) × 200.)
- 1. Garner DM, Garfinkel PE. Handbook of psychotherapy for anorexia nervosa and bulimia. New York: The Guilford Press, 1995.
- 2. Selvini-Palazzoli M. Self-starvation. Haywards Heath, UK: Human Context Books, 1974.
- 3. Becker AE, Grinspoon SK, Klibanski A, Herzog DB. Eating disorders. N Engl J Med 1999; 340: 1092-1098.
- 4. Comerci GD. Medical complications of anorexia nervosa and bulimia nervosa. Med Clin North Am 1990; 74: 1293-1310.
- 5. Greenfeld D, Mickley D, Quinlan D, Roloff P. Hypokalemia in outpatients with eating disorders. Am J Psychiatry 1995; 152: 60-63.
- 6. Sharp CW, Freeman CP. The medical complications of anorexia nervosa. Br J Psychiatry 1993; 162: 452-462.
- 7. Silber TJ, Kass EJ. Anorexia nervosa and nephrolithiasis. J Adolesc Health Care 1984; 5: 50-52.
- 8. Jonat LM, Birmingham CL. Kidney stones in anorexia nervosa: a case report and review of the literature. Eat Weight Disord 2003; 8: 332-335.
- 9. Komori K, Arai H, Gotoh T, et al. A case of ammonium urate urinary stones with anorexia nervosa. Hinyokika Kiyo 2000; 46: 627-629.
- 10. Hara N, Koike H. A case of ammonium urate urinary stone. Hinyokika Kiyo 2004; 50: 351-353.
- 11. Brotman AW, Stern TA, Brotman DL. Renal disease and dysfunction in two patients with anorexia nervosa. J Clin Psychiatry 1986; 47: 433-434.
- 12. Cotran RS. Robbins pathological basis of disease. Philadelphia: WB Saunders Company, 1999.
- 13. Wrong O. Nephrocalcinosis. In: Cameron S, editor. The Oxford textbook of clinical nephrology. Volume 3. Oxford: Oxford University Press, 1992: 1882-1905.
- 14. Kim YG, Kim B, Kim MK, et al. Medullary nephrocalcinosis associated with long-term furosemide abuse in adults. Nephrol Dial Transplant 2001; 16: 2303-2309.
- 15. Tajiri J, Nakayama M, Sato T, et al. Pseudo-Bartter’s syndrome due to furosemide abuse: report of a case and an analytical review of Japanese literature. Jpn J Med 1981; 20: 216-221.
- 16. Markowitz GS, Nasr SH, Klein P, et al. Renal failure due to acute nephrocalcinosis following oral sodium phosphate bowel cleansing. Hum Pathol 2004; 35: 675-684.
- 17. Arimura Y, Tanaka H, Yoshida T, et al. Anorexia nervosa: an important cause of chronic tubulointerstitial nephropathy. Nephrol Dial Transplant 1999; 14: 957-959.
- 18. Abdel-Rahman EM, Moorthy AV. End-stage renal disease (ESRD) in patients with eating disorders. Clin Nephrol 1997; 47: 106-111.





None identified.