Central Australian Aboriginal children have high rates of diarrhoeal disease. In the Northern Territory, diarrhoeal illnesses rank second in the reasons for hospital admission in the 0–12 months and 1–4 years age groups (333.7 and 77.2 per 1000 Indigenous population, respectively).1 The rate in the 0–12 months age group is over 100 times that in non-Indigenous Australians.
International studies indicate that, in some populations, zinc and vitamin A, when used as co-adjunct treatment for diarrhoea in children, can reduce the mortality and morbidity associated with this condition. In developing countries, zinc supplementation has been shown to reduce both the duration and severity of acute diarrhoea.2-9 Results from studies on vitamin A supplementation are more variable, with some studies showing a protective effect.8,10 Such studies suggest that these interventions may provide major benefits to Indigenous children in Australia who have high rates of infectious diseases, malnutrition and poor living conditions.8,10
On the other hand, haematological abnormalities11 and even increased mortality12 after exposure to high doses of zinc have also been reported.
Furthermore, as most previous trials of zinc and vitamin A supplementation for diarrhoea have been community-based (and thus more prone to difficulties with monitoring compliance and conducting follow-up), we cannot assume that their findings would apply to hospitalised children with diarrhoea.
One justification for studying simultaneous treatment with zinc and vitamin A is the evidence that vitamin A is dependent on zinc sufficiency for many of its actions.13 At the time of our study, there were no previous published studies on the effect of combined zinc and vitamin A supplementation in hospitalised children with diarrhoea.
Our aim was to evaluate whether zinc and vitamin A supplementation could reduce the recovery time of Aboriginal children hospitalised for gastroenteritis and reduce the incidence of recurrence of diarrhoea.
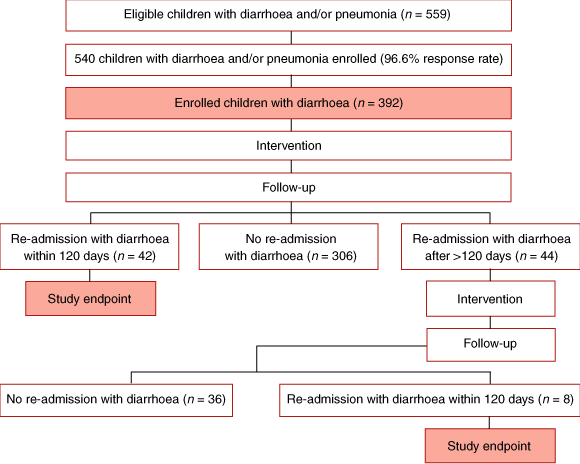
As part of a larger study of pneumonia and diarrhoea in Indigenous children, we conducted a hospital-based, randomised controlled 2 by 2 factorial trial of zinc and vitamin A supplementation in Indigenous children at the Alice Springs Hospital in the Northern Territory. All Aboriginal children under 11 years of age who were admitted to the hospital with pneumonia and/or acute diarrhoea (> 3 loose stools per day) between April 2001 and July 2002 were eligible (Box 1). However, children with bronchiolitis syndrome (wheezing and coryza), chronic lung disease (eg, bronchiectasis, cough lasting more than 2 months), established gastrointestinal disease (eg, short gut syndrome) or neurological disease were excluded. We present here our findings relating to diarrhoea.
Informed consent was obtained from the carer(s) by an Indigenous research officer or a member of the research team before enrolment. Children enrolled were randomly assigned (using a computer-generated permutated block design) within strata of age group (0–12 months and 1–10 years) and disease (pneumonia or diarrhoea), then allocated randomly to one of four treatment regimens (zinc supplementation, vitamin A supplementation, combined zinc + vitamin A supplementation, or placebo). (A randomly generated list to determine next-treatment-group allocation had been provided by the Queensland Institute of Medical Research). A black sticker obscuring the treatment group was removed only after enrolment.
A research team member administered the medication. Active and placebo zinc were similar in appearance but not in taste, while active and placebo forms of vitamin A looked different from one another. Children, their parents and nurses, who recorded daily data, were blinded to the treatment received. Trial medication was commenced within 24 hours of admission.
Based on previous studies,8,14,15 medium to high doses of active medication were chosen: for children under 12 months, vitamin A 50 000 IU (Day 1 and 5) and elemental zinc (zinc sulphate) 20 mg daily for 5 days; for children aged 1–10 years, vitamin A 100 000 IU (Day 1 and 5) and elemental zinc 40 mg daily for 5 days. Children discharged before 5 days did not receive the complete intervention.
Data were collected on standardised forms and outcome variables were recorded daily until discharge. Anthropometric assessment was performed at baseline and discharge. Weight was determined to within 0.1 kg using an electronic scale (baseline weight was measured after hydration); length (for children < 24 months) and height (for those over 24 months) were measured to within 0.1 cm.
All patients were tested for serum potassium and haemoglobin and arterial pH levels. For a sample of 37 children, serum levels of zinc, vitamin A, retinol-binding protein (the transport protein for vitamin A) and albumin were measured at baseline. (These children were chosen arbitrarily, with selection unrelated to disease severity. Budget constraints prevented us from carrying out the tests on all children.) Blood for these tests was collected before the administration of trial medication. Tests followed standard laboratory methods for collection and processing.16
Primary outcome measures were duration of diarrhoeal episode and recurrence of diarrhoea within 120 days. An episode of diarrhoea was defined as at least 24 hours of diarrhoea (> 3 loose stools per day) and considered terminated on the last day of diarrhoea that was followed by at least 24 hours free of diarrhoea. After the first admission for diarrhoea, children were followed up for 120 days for re-admissions for diarrhoea. Any who were re-admitted after 120 days were re-enrolled in the study (Box 1).
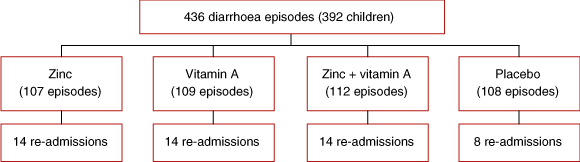
Sample size calculations were based on previous reports and determined a priori. Study power was recalculated when the final sample size was known. With duration of diarrhoea as the endpoint (2-sided test comparing geometric mean number of days), the power of the study to detect a 25%, 20% and 15% reduction in diarrhoea duration (compared with 3.5 days in the placebo group) was 92%, 57% and 45%, respectively (at the 5% significance level). With re-admission for gastroenteritis as the endpoint (2-sided test comparing proportions of children re-admitted), the power of the study to detect a 36% reduction in recurrence among the zinc and vitamin A groups was 80% if 436 episodes (218 in each group) were studied (5% significance level). The study had 90% power to detect a 42% reduction in re-admission for gastroenteritis. Comparing the group receiving both vitamin A and zinc with the placebo group (no vitamin A or zinc), the study had 80% power to detect a 50% reduction in the rate of re-admission.
Data were analysed using SPSS software.17 Basic descriptive summaries were compiled for each treatment group. The Kaplan–Meier method of survival analysis was used to look at time to re-admission (censored at 120 days from Day 1 of the intervention or re-admission date, whichever came earlier). The factorial study design (Box 2) allowed comparison between children who did or did not receive zinc supplementation (ignoring vitamin A supplementation status) and between those who did or did not receive vitamin A supplementation (ignoring zinc supplementation status). All relative risks (RRs) were calculated using generalised estimating equations (Stata’s generalised linear model with the “cluster” option18) to correct for standard errors arising from the fact that not all observations were independent (some children were re-enrolled in the study). All RRs were adjusted for the effect of vitamin A supplementation in the zinc-supplemented group and vice versa. To explore for synergistic effects of receiving both zinc and vitamin A, an interaction term for zinc supplementation and vitamin A supplementation was added to the model.
We used Epi Info19 to calculate z scores. Children were classified as “stunted” (height-for-age ≤ 2 z scores below the mean), “wasted” (weight-for-height/length ≤ 2 z scores below the mean), or “underweight” (weight-for-age ≤ 2 z scores below the mean).20 For continuous normally-distributed variables (eg, number of days in hospital), we reported means and compared groups using a t test for independent samples. For non-normally-distributed data (eg, serum levels of zinc and vitamin A, duration of diarrhoea), we reported medians and used non-parametric tests. Study endpoints for each treatment group were formally analysed on an “intention to treat” basis.21
Of 559 children eligible to be included in the study for either diarrhoea or pneumonia, 540 took part (96.6% response rate). Here we report results related to 436 episodes of diarrhoea among 392 children (44 of the episodes were re-admissions) (Box 1). The demographic and baseline characteristics of patients were similar in the four treatment groups (Box 3 and Box 4).
Children who did not complete the intervention (31.2%) were mostly discharged before 5 days. Of those assigned to vitamin A supplementation, 67.9% of the vitamin A group and 64.2% of the placebo group took two doses (P = 0.42). Of those assigned to zinc supplementation, 90.9% of the zinc group and 88.5% of the placebo group took at least three doses (P = 0.32). None of the children enrolled in the study experienced adverse effects from an intervention. Although zinc sulfate is well known for its metallic taste, about 85% of the children had no problems taking it; 6.8% spat out some and 0.4% vomited.
After intervention, there were very small, non-significant differences in diarrhoea, re-admission with gastroenteritis and weight change (Box 5) between the supplemented and non-supplemented groups for either zinc or vitamin A supplementation. Number of days in hospital was similar for the vitamin A supplemented group compared with the vitamin A placebo group (median 5.0 days for both groups; P = 0.61), and for the zinc supplemented group compared with the zinc placebo group (median 5.0 days for both groups; P = 0.56). Diarrhoea duration after starting supplementation was similar for vitamin A supplementation and for zinc supplementation when compared with relevant placebo groups (median 3.0 days for all groups; P values 0.25 and 0.69, respectively) (Box 5). Similarly, for children taking combined zinc and vitamin A compared with zinc, vitamin A or placebo alone, there were very small differences in diarrhoea duration, rate of re-admission with gastroenteritis and weight change (data not presented), none being statistically significant (RR of re-admission with diarrhoea 1.1 for zinc + vitamin A compared with other groups; 95% CI, 0.6–2.0).
Subgroup analyses of children according to their nutritional status (eg, whether they were stunted or not) did not reveal any significant differences between subgroups. However, these analyses were based on only a small number of children in each cell. There was a slight, non-significant trend towards shorter hospital stay and shorter duration of diarrhoea among stunted children receiving supplementation.
Our study investigated the clinical effect of vitamin A and zinc supplementation in Aboriginal children hospitalised for acute diarrhoea. Overall, when comparing children in the zinc and vitamin A supplemented groups with the respective non-supplemented groups, there was no significant effect on diarrhoea duration, weight gain, time to re-admission with diarrhoea and length of hospital stay. A small, non-significant positive effect of zinc and vitamin A supplements was seen in the subgroup of stunted children. It is worth noting that in Central Australia, children are frequently kept in hospital longer than disease duration because of the geographic isolation of their communities and issues of available family support. Therefore, length of hospital stay may not be a good outcome measure.
The lack of any significant impact of vitamin A supplements on diarrhoeal morbidity is consistent with some previous studies looking at the effect of large doses of vitamin A on early clinical recovery from severe diarrhoea.8,22 Although the baseline vitamin A levels measured in our study were all low, this is unlikely to reflect the true vitamin A status in the population we studied. It is well recognised that acute diarrhoeal illness can be associated with acute falls in measured values of serum vitamin A, which rapidly recover even without supplementation.23,24
At the commencement of our study there were no published studies on the effect of combined vitamin A and zinc supplementation. A recent community-based study in Dhaka, Bangladesh, showed that combined zinc and vitamin A treatment reduced the prevalence of persistent diarrhoea (defined as any diarrhoeas that lasted for at least 14 consecutive days) more than either supplement alone.15 In our study, the lack of effect for zinc was consistent with some existing data25 but inconsistent with a recent meta-analysis.26 In most studies from developing countries, benefits from zinc supplementation have been most evident in zinc-deficient children.27
Although there are no community or hospital data on levels of zinc or vitamin A in the population we studied, our results suggest that these children are not zinc-deficient. A possible explanation for why our results, particularly for zinc, differ from those in other settings is that supplementation is only effective in populations with a low baseline level of these micronutrients. This may be a critical issue in determining which populations may benefit from these interventions. Our study does not address whether Aboriginal children who are malnourished, have low baseline levels of serum vitamin A or zinc, or have chronic rather than acute diarrhoea may benefit. If these are the children most likely to benefit, our study had very little power to detect differences. However, our results suggest a positive effect of vitamin A and zinc supplementation in stunted children, and this should be explored in further research.
Other factors may have contributed to the lack of effect on morbidity in this population. The research team directly involved in the study were not blinded to the vitamin A supplementation. However, given that allocation of treatment was concealed and that the nurses collecting data, the children and their parents were blinded, this source of bias is unlikely.
Our study lacked information on baseline serum zinc and vitamin A levels for all children. If the mean levels of zinc and vitamin A were, by chance, higher in the supplemented groups, this could have biased the results towards the null hypothesis. However, the baseline characteristics of the four treatment groups were very similar. Furthermore, zinc and vitamin A levels among the small sample of children who were tested were similar across the treatment groups. Thus, it is likely that mean levels of zinc and vitamin A were also fairly evenly distributed across the entire sample.
Acknowledging the limitations of our study, including its low power to detect small differences between groups, we conclude that vitamin A and zinc supplementation may not be indicated in the management of acute diarrhoea among hospitalised Aboriginal children living in remote areas of Australia. This finding may not apply to children with malnutrition. Nevertheless, management protocols based on data from developing countries should not be implemented in populations of indigenous children living in developed countries without further supportive evidence. Data on the vitamin A and zinc status as well as nutritional status of study populations will be essential if future trials are to explain different results in different populations.
3 Characteristics of children with gastroenteritis at entry into the trial, by treatment group*†
|
Zinc (n = 107) |
Vitamin A (n = 109) |
Zinc + vitamin A (n = 112) |
Placebo (n = 108) |
P |
||||||||||
Age (months) |
|
|
|
|
|
||||||||||
0–11 |
48 (44.9%) |
53 (48.6%) |
54 (48.2%) |
49 (45.4%) |
0.981 |
||||||||||
12–23 |
42 (39.3%) |
37 (33.9%) |
39 (34.8%) |
40 (37.0%) |
|
||||||||||
≥ 24 |
17 (15.9%) |
19 (17.5%) |
19 (17.0%) |
19 (17.6%) |
|
||||||||||
Sex |
|
|
|
|
|
||||||||||
Male |
52 (48.6%) |
57 (52.3%) |
64 (57.1%) |
63 (58.3%) |
0.450 |
||||||||||
Female |
55 (51.4%) |
52 (47.7%) |
48 (42.9%) |
45 (41.7%) |
|
||||||||||
Household employment |
|
|
|
|
|
||||||||||
Mostly unemployed |
69 (71.9%) |
62 (67.4%) |
74 (74.0%) |
60 (64.5%) |
0.760 |
||||||||||
Even numbers |
14 (14.6%) |
14 (15.2%) |
13 (13.0%) |
19 (20.4%) |
|
||||||||||
Mostly employed |
13 (13.5%) |
16 (17.4%) |
13 (13.0%) |
14 (15.1%) |
|
||||||||||
Place of residence |
|
|
|
|
|
||||||||||
Alice Springs |
30 (28.0%) |
40 (36.7%) |
42 (37.5%) |
44 (40.7%) |
0.248 |
||||||||||
Outside Alice Springs |
77 (72.0%) |
69 (63.3%) |
70 (62.5%) |
64 (59.3%) |
|
||||||||||
Weight-for-age z score |
|
|
|
|
|
||||||||||
≤ –2 (underweight) |
20 (19.0%) |
20 (18.7%) |
16 (14.5%) |
19 (18.3%) |
0.930 |
||||||||||
–1 |
30 (28.6%) |
29 (27.1%) |
33 (30.0%) |
34 (32.7%) |
|
||||||||||
≥ 0 |
55 (52.4%) |
58 (54.2%) |
61 (55.5%) |
51 (49.0%) |
|
||||||||||
Weight-for-height/length z score |
|
|
|
|
|||||||||||
≤ –2 (wasted) |
9 (9.4%) |
15 (15.6%) |
16 (16.3%) |
17 (17.7%) |
0.698 |
||||||||||
–1 |
29 (30.2%) |
27 (28.1%) |
24 (24.5%) |
28 (29.2%) |
|
||||||||||
≥ 0 |
58 (60.4%) |
54 (56.3%) |
58 (59.2%) |
51 (53.1%) |
|
||||||||||
Height-for-age z score |
|
|
|
|
|
||||||||||
≤ –2 (stunted) |
13 (13.4%) |
7 (7.1%) |
6 (6.0%) |
6 (6.3%) |
0.411 |
||||||||||
–1 |
22 (22.7%) |
23 (23.5%) |
19 (19.0%) |
24 (25.0%) |
|
||||||||||
≥ 0 |
62 (63.9%) |
68 (69.4%) |
75 (75.0%) |
66 (68.8%) |
|
||||||||||
Breastfeeding |
|
|
|
|
|
||||||||||
Still breastfeeding |
76 (77.6%) |
70 (73.7%) |
74 (80.4%) |
66 (73.3%) |
0.474 |
||||||||||
Stopped breastfeeding |
5 (5.1%) |
6 (6.3%) |
8 (8.7%) |
10 (11.1%) |
|
||||||||||
Never breastfed |
17 (17.3%) |
19 (20.0%) |
10 (10.9%) |
14 (15.6%) |
|
||||||||||
* Figures (except P values) represent number (%) of children. †Data were missing on breastfeeding (61 children), household employment (55 children), weight-for-height/length z score (50 children), height-for-age z score (45 children) and weight-for-age z score (10 children). |
|||||||||||||||
4 Characteristics of children with gastroenteritis at entry into the trial, by treatment group*†
|
Zinc |
Vitamin A |
Zinc + vitamin A |
Placebo |
P |
||||||||||
Diarrhoea duration before hospital admission |
|||||||||||||||
< 1 day |
26 (27.1%) |
30 (31.6%) |
29 (30.2%) |
29 (31.2%) |
0.942 |
||||||||||
1–2 days |
42 (43.8%) |
35 (36.8%) |
34 (35.4%) |
31 (33.3%) |
|
||||||||||
3–4 days |
20 (20.8%) |
18 (18.9%) |
21 (21.9%) |
24 (25.8%) |
|
||||||||||
≥ 5 days |
8 (8.4%) |
12 (12.6%) |
12 (12.5%) |
9 (9.7%) |
|
||||||||||
Previous episodes of hospitalised diarrhoea |
|||||||||||||||
0 |
70 (69.3%) |
71 (74.7%) |
71 (66.4%) |
79 (78.2%) |
0.241 |
||||||||||
1 |
13 (12.9%) |
15 (15.8%) |
22 (20.6%) |
11 (10.9%) |
|
||||||||||
≥ 2 |
18 (17.9%) |
9 (9.5%) |
14 (13.1%) |
11 (10.9%) |
|
||||||||||
Acidosis |
|
|
|
|
|
||||||||||
pH ≤ 7.35 |
64 (66.7%) |
62 (62.6%) |
62 (58.5%) |
59 (64.1%) |
0.676 |
||||||||||
|
|
|
|
|
|
||||||||||
Serum potassium level |
|
|
|
|
|||||||||||
K+ < 3.5 mmol/L |
58 (57.4%) |
49 (47.1%) |
51 (47.7%) |
49 (49.0%) |
0.417 |
||||||||||
|
|
|
|
|
|
||||||||||
Dehydration |
|
|
|
|
|
||||||||||
Mild (< 5%) |
63 (67.0%) |
64 (68.1%) |
64 (68.8%) |
67 (73.6%) |
0.540 |
||||||||||
Moderate (5%–9%) |
27 (28.7%) |
21 (22.3%) |
25 (26.9%) |
20 (22.0%) |
|
||||||||||
Severe (> 9%) |
4 (4.3%) |
9 (9.6%) |
4 (4.3%) |
4 (4.4%) |
|
||||||||||
Haemoglobin level |
|
|
|
|
|
||||||||||
Anaemia (< 110 g/L) |
53 (53.5%) |
52 (49.5%) |
37 (35.9%) |
51 (50.0%) |
0.060 |
||||||||||
* Figures (except P values) represent number (%) of children. †Data were missing on clinical level of dehydration (64 children), acidosis (43 children), previous hospitalised episodes of diarrhoea (32 children), haemoglobin level (27 children) and potassium level (24 children). |
|||||||||||||||
5 Diarrhoea duration after intervention, proportion of patients re-admitted, and weight changes in groups supplemented with zinc or vitamin A*†
|
Vitamin A supplementation |
Zinc supplementation |
|||||||||||||
|
Yes |
No |
RR (95% CI) |
Yes |
No |
RR (95% CI) |
|||||||||
Diarrhoea duration after starting intervention |
|||||||||||||||
≤ 3 days |
134 (64.1%) |
134 (66.0%) |
1.0 |
136 (65.7%) |
132 (64.4%) |
1.0 |
|||||||||
4–6 days |
61 (29.2%) |
58 (28.6%) |
1.1 (0.7–1.6) |
58 (28.0%) |
61 (29.8%) |
0.9 (0.6–1.4) |
|||||||||
≥ 7 days |
14 (6.7%) |
11 (5.4%) |
1.3 (0.6–2.9) |
13 (6.3%) |
12 (5.9%) |
1.1 (0.5–2.4) |
|||||||||
P (trend) |
|
|
0.593 |
|
|
0.882 |
|||||||||
Re-admission with gastroenteritis |
|
|
|
||||||||||||
No |
193 (87.3%) |
193 (89.8%) |
1.0 |
191 (87.2%) |
195 (89.9%) |
1.0 |
|||||||||
Yes |
28 (12.7%) |
22 (10.2%) |
1.2 (0.7–2.1) |
28 (12.7%) |
22 (10.2%) |
1.3 (0.8–2.1) |
|||||||||
Change in weight during hospital stay |
|
|
|
||||||||||||
Weight gain |
175 (81.0%) |
168 (81.2%) |
1.0 |
176 (82.6%) |
167 (79.5%) |
1.0 |
|||||||||
Weight loss or no change |
41 (19.0%) |
39 (18.8%) |
1.0 (0.9–1.1) |
37 (17.4%) |
43 (20.5%) |
1.0 (0.9–1.1) |
|||||||||
RR = relative risk. * Figures (except RRs and P values) represent number (%) of children. †Data were missing on weight change (13 children) and diarrhoea duration (24 children). |
|||||||||||||||
Received 18 November 2004, accepted 8 April 2005
- Patricia C Valery1
- David M Purdie2
- Paul J Torzillo3
- Peter A Stewart4
- Naomi C Boyce5
- Anne B Chang6
- Andrew V White7
- Gavin R Wheaton8
- John Wakerman9
- 1 Population Studies and Human Genetics, Queensland Institute of Medical Research, Herston, QLD.
- 2 Royal Prince Alfred Hospital and University of Sydney, Camperdown, NSW.
- 3 Northern Territory Clinical School, Flinders University, Alice Springs, NT.
- 4 Community Health Services — Remote, NT Department of Heath and Community Services, Alice Springs, NT.
- 5 Alice Springs Hospital, Alice Springs, NT.
- 6 Centre for Remote Health, Flinders University and Charles Darwin University, Alice Springs, NT.
We wish to thank the children and their families for participating in the study, and Mrs Valerie Logan for technical support. The Sylvia and Charles Viertel Charitable Foundation (A Chang Clinical Investigatorship), Flinders University Research Centre and Flinders University Northern Territory Clinical School provided funding for data collection but had no role in study design, analysis, interpretation and publication.
None identified.
- 1. d’Espaignet E, Kennedy K, Paterson B, Measey M. From infancy to young adulthood: health status in the Northern Territory. Darwin: Territory Health Services, 1998.
- 2. Bhatnagar S, Bahl R, Sharma PK, et al. Zinc with oral rehydration therapy reduces stool output and duration of diarrhea in hospitalized children: a randomized controlled trial. J Pediatr Gastroenterol Nutr 2004; 38: 34-40.
- 3. Al-Sonboli N, Gurgel RQ, Shenkin A, et al. Zinc supplementation in Brazilian children with acute diarrhoea. Ann Trop Paediatr 2003; 23: 3-8.
- 4. Sazawal S, Black RE, Bhan MK, et al. Zinc supplementation in young children with acute diarrhea in India. N Engl J Med 1995; 333: 839-844.
- 5. Penny ME, Peerson JM, Marin RM, et al. Randomized, community-based trial of the effect of zinc supplementation, with and without other micronutrients, on the duration of persistent childhood diarrhea in Lima, Peru. J Pediatr 1999; 135 (2 Pt 1): 208-217.
- 6. Roy SK, Tomkins AM, Akramuzzaman SM, et al. Randomised controlled trial of zinc supplementation in malnourished Bangladeshi children with acute diarrhoea. Arch Dis Child 1997; 77: 196-200.
- 7. Roy SK, Tomkins AM, Mahalanabis D, et al. Impact of zinc supplementation on persistent diarrhoea in malnourished Bangladeshi children. Acta Paediatr 1998; 87: 1235-1239.
- 8. Faruque AS, Mahalanabis D, Haque SS. Double-blind, randomized controlled trial of zinc or vitamin A supplementation in young children with acute diarrhoea. Acta Paediatr 1999; 88: 154-160.
- 9. Hidayat A, Achadi A, Sunoto, Soedarmo S. The effect of zinc supplementation in children under three years of age with acute diarrhoea in Indonesia. Med J Indones 1998; 7: 237-241.
- 10. Si NV, Grytter C, Vy NN, et al. High dose vitamin A supplementation in the course of pneumonia in Vietnamese children. Acta Paediatr 1997; 86: 1052-1055.
- 11. Porea TJ, Belmont JW, Mahoney DH Jr. Zinc-induced anemia and neutropenia in an adolescent. J Pediatr 2000; 136: 688-690.
- 12. Doherty CP, Sarkar MA, Shakur MS, et al. Zinc and rehabilitation from severe protein-energy malnutrition: higher-dose regimens are associated with increased mortality. Am J Clin Nutr 1998; 68: 742-748.
- 13. Christian P, West KP Jr. Interactions between zinc and vitamin A: an update. Am J Clin Nutr 1998; 68 (2 Suppl): 435S-441S.
- 14. Bhutta ZA, Bird SM, Black RE, et al. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: pooled analysis of randomized controlled trials. Am J Clin Nutr 2000; 72: 1516-1522.
- 15. Rahman MM, Vermund SH, Wahed MA, et al. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: randomised double blind controlled trial. BMJ 2001; 323: 314-318.
- 16. Central Sydney Laboratory Service. Fact sheet for zinc (plasma). Sydney: Biochemistry Department, Royal Prince Alfred Hospital, 2004. Available at: http://www.cs.nsw.gov.au/csls (accessed Apr 2005).
- 17. SPSS for Windows. Release 13.0. Chicago, Ill: SPSS Inc., 2004.
- 18. Stata statistical software. Intercooled Stata 8.0 for Windows. College Station, Tex: Stata Corporation, 2003.
- 19. Center for Disease Prevention. Epi Info 2002. Atlanta, Ga: US Department of Health and Human Services, 2002.
- 20. World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva: WHO, 1995.
- 21. Meinert CL. Clinical trials: design, conduct and analysis. New York: Oxford University Press, 1986: 185-195.
- 22. Rollins NC, Filteau SM, Elson I, Tomkins AM. Vitamin A supplementation of South African children with severe diarrhea: optimum timing for improving biochemical and clinical recovery and subsequent vitamin A status. Pediatr Infect Dis J 2000; 19: 284-289.
- 23. Mitra AK, Alvarez JO, Wahed MA, et al. Predictors of serum retinol in children with shigellosis. Am J Clin Nutr 1998; 68: 1088-1094.
- 24. Stephensen CB, Alvarez JO, Kohatsu J, et al. Vitamin A is excreted in the urine during acute infection. Am J Clin Nutr 1994; 60: 388-392.
- 25. Bhutta ZA, Nizami SQ, Isani Z. Zinc supplementation in malnourished children with persistent diarrhea in Pakistan. Pediatrics 1999; 103: e42.
- 26. Black RE. Zinc deficiency, infectious disease and mortality in the developing world. J Nutr 2003; 133 (5 Suppl 1): 1485S-1489S.
- 27. Sazawal S, Black RE, Bhan MK, et al. Zinc supplementation reduces the incidence of persistent diarrhea and dysentery among low socioeconomic children in India. J Nutr 1996; 126: 443-450.





Abstract
Objective: To evaluate the role of zinc and vitamin A supplementation in the recovery of Indigenous children hospitalised for acute diarrhoea.
Design: A randomised controlled 2 by 2 factorial trial of supplementation with zinc and vitamin A.
Setting and participants: Aboriginal children (aged < 11 years) hospitalised for acute diarrhoea at Alice Springs Hospital, Northern Territory, April 2001–July 2002.
Main outcome measures: Duration of diarrhoeal illness; re-admission for diarrhoeal illness within 120 days.
Results: Our study involved 392 Aboriginal children with 436 episodes of diarrhoea. Supplementation with zinc, vitamin A, or combined zinc and vitamin A had no significant effect on duration of diarrhoea or rate of re-admission compared with placebo. Median diarrhoea duration after starting supplementation was 3.0 days for the vitamin A and zinc supplemented and placebo groups (P values 0.25 and 0.69, respectively). The number of re-admissions did not differ significantly between those receiving vitamin A or zinc and the relevant placebo groups (relative risk [95% CI], 1.2 [0.7–2.1] and 1.3 [0.8–2.1], respectively).
Conclusion: Vitamin A and zinc supplementation may not be indicated for in-hospital management of acute diarrhoeal disease in Aboriginal children living in remote areas. This finding may not apply to children with malnutrition, for whom other studies suggest a benefit. Larger trials incorporating more comprehensive data on the vitamin A and zinc status as well as nutritional status of study populations might help to explain the different results in different populations.