Heroin overdose is a major cause of death in some countries,1,2 and until recently rates of death from overdose have been increasing in Australia.2-4 Many lives are saved in the community by the administration of naloxone to patients with suspected opioid overdose by ambulance officers.5 In Victoria, this is usually administered by the intramuscular (IM) route. The intravenous (IV) route is only used in specific circumstances, guided by clinical practice guidelines.6
Administering naloxone by injection (IM or IV) exposes healthcare workers, including ambulance officers, to a degree of risk, as many patients with heroin overdose carry blood-borne viruses that may be transmitted by needlestick injuries.
The sublingual and oral routes for administering naloxone have been found to be less effective than the IM and IV routes because of delayed onset of action.7-8 Pharmacological data from animal studies suggest that naloxone is 100% bioavailable through the nasal mucosa, with onset of action and plasma bioavailability curves that are indistinguishable from those for the IV route.9 Compared with intranasal (IN) and IV naloxone administration, IM naloxone administration shows delayed onset of action.10 IN naloxone has been shown to be effective in detecting opioid dependence10 and to be as effective as the IV route for the reversal of opioid effects in dependent patients.11
To date, the evaluation of administering naloxone intranasally for the emergency treatment of opiate overdose has been limited. An earlier pilot study of six cases by two of us supports its effectiveness in treating acute opiate overdose.12 That series of six cases found that all patients had return of adequate spontaneous respiration within two minutes of administration, with a median of 50 seconds. Another prospective cohort study of 30 patients given IN naloxone as first-line treatment for suspected opiate toxicity reported that 91% of patients had a significant improvement in conscious state. That study predicted that 64% of patients would not have required IV access in the prehospital setting with initial IN naloxone treatment.13 However, that study’s findings are limited by the relatively small sample size, the non-randomised consecutive sampling technique and the subjectivity of the outcome.
The aim of our study was to determine the effectiveness of IN naloxone compared with IM naloxone for patients with acute respiratory depression secondary to suspected opiate overdose treated in the prehospital setting.
This prospective, randomised, unblinded study of patients who required treatment with naloxone for suspected heroin overdose by ambulance officers was conducted in rural and metropolitan Victoria between 5 January 2002 and 19 December 2003. Patients were randomly allocated to receive either 2 mg IN naloxone by means of a mucosal atomiser or 2 mg IM naloxone, in addition to basic life support governed by clinical practice guidelines.6 The study was approved by the Royal Melbourne Hospital Human Research and Ethics Committee and a requirement for written patient consent was waived. All patients were informed of their enrolment in the study by way of a study information brochure when they regained consciousness .
This study was conducted by paramedics of the Metropolitan Ambulance Service (MAS) and Rural Ambulance Victoria (RAV). Together, these services provide almost 100% of emergency ambulance response in Victoria.
Paramedics enrolled patients into the study in whom opiate overdose was suspected, who had fewer than 10 respirations per minute and were not rousable. Random allocation was by random number allocation, with the treatment protocol contained in a sealed envelope that was opened after patient eligibility was determined. Patients received either 2 mg IN naloxone (1 mg into each nostril) by means of a disposable mucosal atomiser attached to a syringe (Mucosal Atomiser Device; Wolfe-Tory Medical) or 2 mg IM naloxone. A 2 mg dose was chosen because previous studies had demonstrated IN naloxone effectiveness at this dose.13 In addition to naloxone, all patients received standard respiratory support and standard supportive care.
All patients who failed to respond within 8 minutes of initial naloxone administration were given a further 0.8 mg of IM naloxone, and paramedics were instructed to continue to treat patients according to standard protocols and to transport patients to hospital when appropriate. All paramedics received instruction about the study and the use of the atomiser device.
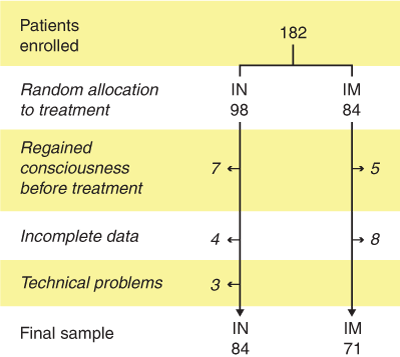
Of the 182 patients initially enrolled in the study, 27 patients were not included in the final cohort (see Box 1), leaving 155 patients in the final sample, 71 of whom received IM naloxone and 84 of whom received IN naloxone.
Most patients were men (111; 72%), and ranged in age from 13 to 57 years (median, 28 years). More patients were treated in public places such as a park or street than in private residences (93 [60%] versus 62 [40%]). For 65 patients (42%) paramedics recorded suspecting other drugs (eg, benzodiazepines, paracetamol) or alcohol consumption in conjunction with the opioid overdose. Needle marks were observed for 96 patients (62%), and a friend or family member was with the patient when the ambulance arrived in 105 cases (67%).
The IN and IM groups were similar for age, sex and suspicion of other drugs or alcohol taken (Box 2). More patients in the IN group were treated in the street.
Patients who received IM naloxone responded faster than the IN group with respect to time of respirations greater than 10 per minute (mean of 6 min [95% CI, 5–7 min] for IM group v mean of 8 min [95% CI, 7–8 min] for IN group; P = 0.006, log rank,Box 3a). Time to GCS score greater than 11 was not significantly different (P = 0.376, log rank,Box 3b). The IM group was more likely to have spontaneous respirations within 8 minutes (82% for the IM group v 63% for the IN group; P = 0.0163; odds ratio [OR], 2.6; 95% CI, 1.2–5.5). The difference in the proportions requiring rescue naloxone (13% for the IM group v 26% for the IN group; P = 0.0558; OR, 2.4; 95% CI, 1.0–5.7)] and having a GCS score greater than 11 at 8 minutes (72% for the IM group v 57% for the IN group; P = 0.0829; OR, 1.9; 95% CI, 0.98–3.7) did not reach statistical significance. There were no major adverse events for either group, but those who received IM naloxone were more likely to experience a minor adverse effect of treatment than those who received IN treatment (21% for the IM group v 12% for the IN group; P = 0.1818; see Box 4). The difference in agitation/irritation between the groups was particularly notable (13% for the IM group v 2% for the IN group; P = 0.0278; see Box 4). Sixty-two of the 84 patients allocated to the IN group (74%) did not require additional therapy.
In this study, IN naloxone was found to be sufficient to reverse the effects of acute opiate toxicity in most patients (74%) treated in this way. This percentage is somewhat lower than previously reported13 and than our pilot study findings,12 but still considerable. Therefore, in the context of appropriate respiratory support and the availability of additional response if required, the effectiveness of IN naloxone appears sufficient to warrant its use as a first-line therapy for acute opiate toxicity in the prehospital setting.
The IN route has been considered for use with a variety of other medications, including fentanyl, midazolam and nitroglycerine.14-16 The benefits of IN administration include ease of access and reduced needlestick exposure. Typically, patients treated in cases of suspected opioid overdose are injecting drug users, in whom the prevalence of blood-borne viruses such as HIV and hepatitis B and C is high.17,18 Treating such patients presents occupational safety issues for healthcare professionals such as paramedics because of an increased risk of exposure to blood-borne infections through needlestick injury. Our findings suggest that IN naloxone could be used as first-line treatment for patients with suspected opiate overdose, which would reduce this risk to healthcare personnel.
In our study, IM administration of naloxone resulted in slightly faster response times and a trend towards a lower requirement for secondary naloxone treatment than IN administration. The reasons for these differences are unclear, as available pharmacokinetic data from animal studies suggest equivalent bioavailability. One possible explanation for the observed difference is the IN preparation that was used. There is currently no standard preparation of naloxone for IN administration. We used 5 mL of the available injectable preparation administered by an atomiser. This volume of fluid may have been too large for ready absorption by the surface of the nasal passages, thereby resulting in a reduced effect. In contrast, a previous report used the same dose, but in a lower volume (2 mg/2 mL solution; 1 mL per nostril), and reported a 91% (10/11) response rate to IN naloxone alone.13 It is possible that the differences in responses observed between our study and the earlier one may be due, at least in part, to differences in drug formulation.
An unexpected finding was the differences in rates of agitation/irritation after naloxone treatment between groups, with patients who received IM treatment showing higher rates than those who received IN treatment. This may be explained by the faster response times observed in our study and differences in rates of absorption,10 and may be an advantage of the IN route.
In our study, most patients (67%) were in the presence of a friend or relative before paramedics arrived. Similar findings have been documented previously,19-22 and suggest that there is often opportunity for intervention before ambulance attendance (eg, expired-air resuscitation).23 One suggested strategy for preventing opioid-overdose-related deaths has been to make naloxone more widely available in the community.24-29 Our findings suggest that naloxone for IN use may be an appropriate form in which this drug could be made more widely available, as it has significant advantages over other forms. An IN form of naloxone would eliminate the need for needles, thereby reducing risks of blood-borne virus transmission. Additionally, the requirement for training and the secure storage of both used and unused syringes and needles could be minimised. A potential disadvantage of IN naloxone shown in our study is the slower response compared with the IM route. However, if the average time for an ambulance to arrive is 8 minutes, availability of naloxone via peers, family or community workers could potentially shorten the period of hypoxia experienced by patients with opioid overdose. This finding also highlights the need for accompanying education about, firstly, the need to call an ambulance immediately, and secondly, about airway management and assisted breathing before an ambulance arrives, in case the patient is one of the 26% who require additional therapy and basic life-support measures. While programs for the wider provision of naloxone have been operating in some jurisdictions for some time, to our knowledge there have been no confirmatory studies of the safety and effectiveness of naloxone administration by peers or family in the community.30 For reasons of practicability and safety, the wider distribution of naloxone within the community may be best undertaken with a specifically formulated naloxone preparation designed for IN administration.
Our study has some limitations. The sample size was smaller than anticipated, as heroin overdose rates in Melbourne during the study period were dramatically reduced from previously observed rates.31 Nevertheless, it had adequate power to detect key statistically significant differences in the primary outcomes. On the other hand, power to detect significant differences in secondary outcomes (eg, requirement for rescue naloxone) was low. Data were collected from patient case records and, as such, depended on the completeness and accuracy of these documents. While paramedics were asked to enrol all patients in whom opioid overdose was suspected and whom they anticipated treating with naloxone, it is possible that eligible patients were missed. A comparison with a simultaneous but independent database of drug-related ambulance attendances in Melbourne32 suggests that less than 5% of patients were missed for study enrolment. This small percentage of cases was unlikely to have resulted from any form of systematic bias. Finally, as naloxone is an opioid antagonist, opioid load (ie, the amount of heroin used) may have an effect on both response rate and time. We were unable to control for opioid load in this study.
2 Comparison of demographic and other data for patients given naloxone intramuscularly and intranasally
Variable |
Intramuscular naloxone |
Intranansal naloxone |
P |
||||||||||||
Median age (range) |
30 (16–57) |
28 (13–52) |
0.7111 |
||||||||||||
Males (%) |
52 (73%) |
59 (70%) |
0.8167 |
||||||||||||
Location |
|
|
0.0194 |
||||||||||||
Street |
14 (20%) |
32 (38%) |
|
||||||||||||
Flat or house |
32 (45%) |
36 (43%) |
|
||||||||||||
Car |
8 (11%) |
6 (7%) |
|
||||||||||||
Train |
5 (7%) |
0 |
|
||||||||||||
Other |
12 (17%) |
10 (12%) |
|
||||||||||||
Transported to hospital |
15 (21%) |
14 (17%) |
0.6138 |
||||||||||||
Suspicion of other drugs/alcohol |
28 (39%) |
37 (44%) |
0.6778 |
||||||||||||
3 Response times for the groups given naloxone intranasally and intramuscularly in terms of return of spontaneous respiration and Glasgow Coma Scale score
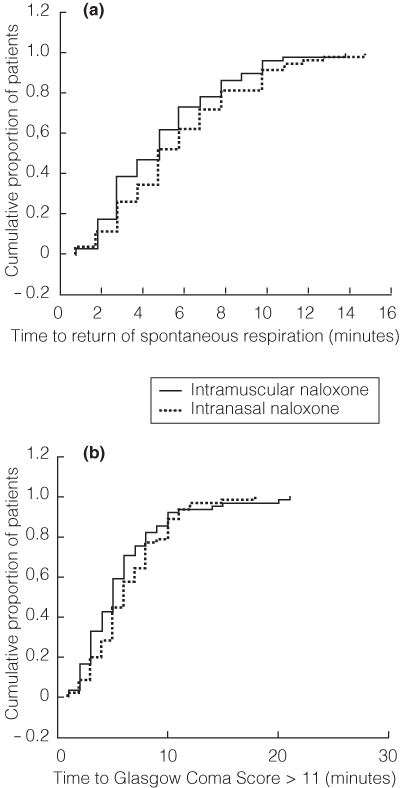
Proportion of each group with (a) respiratory rate > 10 per minute, and (b) with Glasgow Coma Scale score > 11 per minute.
4 Adverse events after naloxone
Adverse event |
No. in intramuscular naloxone group |
No. in intranansal naloxone group |
|||||||||||||
Agitation and/or irritation |
10 |
2 |
|||||||||||||
Nausea and/or vomiting |
4 |
6 |
|||||||||||||
Headache |
2 |
0 |
|||||||||||||
Tremor |
1 |
1 |
|||||||||||||
Sweating |
0 |
1 |
|||||||||||||
Received 2 July 2004, accepted 21 October 2004
- Anne-Maree Kelly1
- Debra Kerr2
- Zeff Koutsogiannis3
- Paul Dietze4
- Ian Patrick5
- Tony Walker6
- 1 Western Hospital, Melbourne, VIC.
- 2 Turning Point Alcohol and Drug Centre, Melbourne, VIC.
- 3 Metropolitan Ambulance Service, Melbourne, VIC.
- 4 Rural Ambulance Victoria, Ballarat, VIC.
This study was supported by a grant from the William Buckland Foundation, and we thank the Foundation for its support. We also thank Mr Bill Barger and Mr Greg Cooper (Metropolitan Ambulance Service) and Mr Paul McElwee (Turning Point Alcohol and Drug Centre) for assistance with data collection, as well as the paramedics involved for their enthusiasm and professionalism.
None identified.
- 1. Oxman G, Kowalski S, Drapela L, et al. Heroin overdose deaths — Multnomah County, Oregon, 1993–1999. MMWR Morb Mortal Wkly Rep 2000; 49: 633-636.
- 2. Hall WD, Degenhardt LJ, Lynskey MT. Opioid overdose mortality in Australia, 1964–1997: birth-cohort trends. Med J Aust 1999; 171: 34-37.
- 3. Dietze P, Fry C, Rumbold G, Gerostamoulos J. The context, management and prevention of heroin overdose in Victoria, Australia: the promise of a diverse approach. Addict Res Theory 2001; 9: 437-458.
- 4. Zador D, Sunjic S, Darke S. Heroin-related deaths in New South Wales, 1992: toxicological findings and circumstances. Med J Aust 1996; 164: 204-207. <MJA full text>
- 5. Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med 1996; 3: 660-667.
- 6. Metropolitan Ambulance Service, Rural Ambulance Victoria. Clinical practice guideline: overdose of narcotic drugs. Version 1, April 2001. Available at: www.ambulance-vic.com.au/downloads/clinical_practices_guide/47_CPG_A0802_Overdose_of_Narcotic_Drugs_010401.pdf (accessed Nov 2004).
- 7. Maio RF, Gaukel B, Freeman B. Intralingual naloxone injection for narcotic-induced respiratory depression. Ann Emerg Med 1987; 16: 572-573.
- 8. Preston KL, Bigelow GE, Liebson IA. Effects of sublingually given naloxone in opioid-dependent human volunteers. Drug Alcohol Depend 1990; 25: 27-34.
- 9. Hussain AA, Kimura R, Huang CH. Nasal absorption of naloxone and buprenorphine in rats. Int J Pharm 1984; 21: 233.
- 10. Loimer N, Hofmann P, Chaudhry HR. Nasal administration of naloxone for detection of opiate dependence. J Psychiatr Res 1992; 26: 39-43.
- 11. Loimer N, Hofmann P, Chaudhry HR. Nasal administration of naloxone is as effective as the intravenous route in opiate addicts. Int J Addict 1994; 29: 819-827.
- 12. Kelly AM, Koutsogiannis Z. Intranasal naloxone for life threatening opioid toxicity. Emerg Med J 2002; 19: 375.
- 13. Barton ED, Ramos J, Colwell C, et al. Intranasal administration of naloxone by paramedics. Prehosp Emerg Care 2002; 6: 54-58.
- 14. Ralley FE. Intranasal opiates: old route for new drugs. Can J Anaesth 1989; 36: 491-493.
- 15. Kendall JL, Reynolds M, Goldberg R. Intranasal midazolam in patients with status epilepticus. Ann Emerg Med 1997; 29: 415-417.
- 16. Mahajan RP, Grover VK, Sharma SL, Singh H. Intranasal nitroglycerin and intraocular pressure during general anesthesia. Anesth Analg 1988; 67: 631-636.
- 17. Crofts N, Jolley D, Kaldor J, et al. Epidemiology of hepatitis C virus infection among injecting drug users in Australia. J Epidemiol Commun Health 1997; 51: 692-697.
- 18. Davoli M, Perucci CA, Rapiti E, et al. A persistent rise in mortality among injection drug users in Rome, 1980 to 1991. Am J Public Health 1997; 87: 851-853.
- 19. McGregor C, Darke S, Ali R, Christie P. Experience of non-fatal overdose among heroin users in Adelaide, Australia: circumstances and risk perceptions. Addiction 1998; 93: 701-711.
- 20. Darke S, Ross J, Hall W. Overdose among heroin users in Sydney, Australia II: responses to overdoses. Addiction 1996; 91: 413-417.
- 21. Darke S, Ross J, Hall W. Overdose among heroin users in Sydney, Australia I: prevalence and correlates of non-fatal overdose. Addiction 1996; 91: 405-411.
- 22. Strang J, Powis B, Best D, et al. Preventing opiate overdose fatalities with take-home naloxone: pre-launch study of possible impact and acceptability. Addiction 1999; 94: 199-204.
- 23. Dietze P, Cantwell K, Burgess S. Bystander resuscitation attempts at heroin overdose: does it improve outcomes? Drug Alcohol Depend 2002; 67: 213-218.
- 24. Seal KH, Downing M, Kral AH, et al. Attitudes about prescribing take-home naloxone to injection drug users for the management of heroin overdose: a survey of street-recruited injectors in the San Francisco Bay Area. J Urban Health 2003; 80: 291-301.
- 25. Dettmer K, Saunders B, Strang J. Take home naloxone and the prevention of deaths from opiate overdose: two pilot schemes. BMJ 2001; 322: 895-896.
- 26. Coffin PO, Fuller C, Vadnai L, et al. Preliminary evidence of health care provider support for naloxone prescription as overdose fatality prevention strategy in New York City. J Urban Health 2003; 80: 288-290.
- 27. Darke S, Hall W. The distribution of naloxone to heroin users. Addiction 1997; 92: 1195-1199.
- 28. Bigg D. Data on take home naloxone are unclear but not condemnatory. BMJ 2002; 324: 678.
- 29. Lenton SR, Hargreaves KM. Should we conduct a trial of distributing naloxone to heroin users for peer administration to prevent fatal overdose? Med J Aust 2000; 173: 260-263. <MJA full text>
- 30. Hargreaves K, Lenton S. Naloxone for overdose: consideration of a trial of naloxone provision for peer or worker administration in Victoria. Perth: Australia. National Drug Research Institute, 2003.
- 31. Cvetkovski S, Dietze P, McElwee P. Non-fatal heroin overdose in Melbourne. Trends in heroin overdose events attended by ambulances in Melbourne: June 1998–June 2002. Monthly report no. 37. April Report. Melbourne: Turning Point Alcohol and Drug Centre, 2002.
- 32. Dietze P, Cvetkovski S, Rumbold G, Miller P. Ambulance attendance at heroin overdose in Melbourne: the establishment of a database of ambulance service records. Drug Alcohol Rev 2000; 19: 27-33.





Abstract
Objective: To determine the effectiveness of intranasal (IN) naloxone compared with intramuscular (IM) naloxone for treatment of respiratory depression due to suspected opiate overdose in the prehospital setting.
Design: Prospective, randomised, unblinded trial of either 2 mg naloxone injected intramuscularly or 2 mg naloxone delivered intranasally with a mucosal atomiser.
Participants and setting: 155 patients (71 IM and 84 IN) requiring treatment for suspected opiate overdose and attended by paramedics of the Metropolitan Ambulance Service (MAS) and Rural Ambulance Victoria (RAV) in Victoria.
Main outcome measures: Response time to regain a respiratory rate greater than 10 per minute. Secondary outcome measures were proportion of patients with respiratory rate greater than 10 per minute at 8 minutes and/or a GCS score over 11 at 8 minutes; proportion requiring rescue naloxone; rate of adverse events; proportion of the IN group for whom IN naloxone alone was sufficient treatment.
Results: The IM group had more rapid response than the IN group, and were more likely to have more than 10 spontaneous respirations per minute within 8 minutes (82% v 63%; P = 0.0173). There was no statistically significant difference between the IM and IN groups for needing rescue naloxone (13% [IM group] v 26% [IN group]; P = 0.0558). There were no major adverse events. For patients treated with IN naloxone, this was sufficient to reverse opiate toxicity in 74%.
Conclusion: IN naloxone is effective in treating opiate-induced respiratory depression, but is not as effective as IM naloxone. IN delivery of naxolone could reduce the risk of needlestick injury to ambulance officers and, being relatively safe to make more widely available, could increase access to life-saving treatment in the community.