One in 20 Australians will develop colorectal cancer (CRC),1 and almost half of these people will die from the disease.2 Survival from CRC is highly stage-dependent. Localised disease has a 94% 5-year survival rate;3 however, using current approaches, only 35% of CRC is diagnosed at this stage.4 Screening to detect presymptomatic individuals in the at-risk age group reduces CRC mortality.5 In addition, nearly all CRCs arise from adenomatous polyps; these typically grow slowly, with a median time to development of cancer of 10 years. As a result, removal of polyps through endoscopic polypectomy may reduce CRC risk by up to 90% in those screened.6
Although CRC screening is desirable and advocated by expert groups,5 it is not widespread. The most appropriate initial screening test — faecal occult blood testing (FOBT), flexible sigmoidoscopy, or colonoscopy — remains a subject of debate.5
Because the success of screening programs hinges on participation rates and compliance, methods for recruitment of participants need to be established. Participation in CRC screening using FOBT has been reported to improve when subjects are invited by their own general practitioners.7,8 However, less is known about participation in colonoscopic screening programs. Therefore, we explored participation in colonoscopic screening by comparing two recruitment strategies: selection of subjects drawn from a sample of general practice databases, followed by invitation by the subject’s own GP, versus invitations by the investigators to subjects drawn at random from the electoral roll.
Recruitment for the study commenced in May 2002 and closed in December 2003, and the final colonoscopy was performed in January 2004.
Electoral roll arm: From the Australian Electoral Commission, we obtained a random sample of residents of the Australian Capital Territory aged 55–74 years. This sample was cross-referenced against the ACT Cancer Registry, and people with a known diagnosis of cancer were excluded. Invitation letters containing simple information about CRC and colonoscopy and signed by the study investigators were mailed to the selected subjects.
General practice arm: Six general practices, geographically spread to best represent the ACT, were recruited to the study. Participating GPs then selected from their practice databases ACT residents aged 55–74 years whom they believed met eligibility criteria (identical to eligibility criteria to be used at Visit 1; see Box 1). An invitation letter identical to that used in the electoral roll arm, but also signed by the invitee’s GP, was mailed.
Reminders: For both arms, a reminder letter was mailed if an invitee did not reply after 4 weeks. We telephoned those invitees who did not respond to the second letter after a further 4 weeks, or who declined without reason. We invited all who declined to complete a non-participant questionnaire.
We contacted respondents by phone or mail, according to their preference, and arranged Visit 1 at the Gastroenterology Unit, Canberra Hospital. At this visit, an information sheet was provided and written consent for the study was obtained. We asked participants about their medical history, including medications, family history of cancer, and previous screening behaviour. Eligibility criteria were assessed (Box 1).
Participants were allocated according to preference to one of three endoscopy centres: Canberra Hospital, Calvary Hospital, or Mugga Wara Endoscopy Centre. All endoscopists were accredited by the Conjoint Committee of the Gastroenterological Society of Australia, the Royal Australasian College of Physicians and the Royal Australasian College of Surgeons for Recognition of Endoscopy Training.
All colonoscopy participants underwent a physical examination to record their fitness for sedation.
GP sedationists or nurse sedationists administered sedation with a combination of fentanyl, midazolam and propofol. Colonoscopy was performed with attention to quality recommendations.10
Participants attended a third time to receive results of the colonoscopy and results of histopathology where relevant. Recommendations for post-polypectomy surveillance were made according to National Health and Medical Research guidelines.11 We sent a letter to the participant’s GP summarising the results and a recommendation for surveillance.
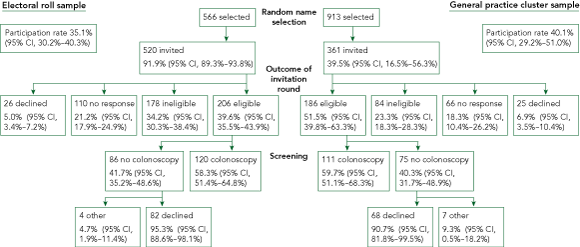
In the electoral roll arm, 566 people were selected. Forty-six were excluded for being on the ACT Cancer Registry, so 520 invitations were sent, resulting in 120 colonoscopies being performed (Box 2). Thus, for every four subjects invited, one screening colonoscopy was performed. In the general practice arm, 913 names were selected from the GPs’ databases, leading to 361 invitations after review of patients’ details, and resulting in 111 colonoscopies (Box 2). Thus, for every three invitations, one screening colonoscopy was performed. This difference in proportions was significant (P = 0.025).
After name selection in the general practice sample, 60% (552/913) were not invited; 309 (56%) of those not invited were classified as “archived”, as their files had been archived after 3 years of inactivity. These selections could not be electronically filtered at the time of name selection, but were not retrievable; consequently, it was not known whether these patients had changed GPs. GPs had various archiving practices, with two practices contributing 271 (88%) of these 309 files. Three of the six general practices required extensive manual reviewing of records to select people for invitation.
Non-response rates were similar between the two arms, with 21.2% of the electoral roll sample and 18.3% of the general practice sample not responding to either invitation letter or telephone call (Box 2). The proportions responding but declining participation were also similar (electoral roll arm, 5.0%; general practice arm, 6.9%).
The sample in the general practice arm was pre-screened for eligibility, whereas the electoral roll arm had limited pre-screening via cancer registry matching. As a result, a higher proportion of the electoral roll invitees were ineligible for participation (electoral roll arm, 34.2%; general practice arm, 23.3%; P < 0.001). The major reason for ineligibility in both arms was previous colonoscopy, accounting for more than 50% (Box 3). Taken across the electoral roll sample of invitees, a conservative estimate is that 19% (95% CI, 15%–22%) of people aged between 55 and 74 years in the ACT had a colonoscopy before the study.
In the general practice arm, 15.6% (84/270) of GPs’ medical records did not contain patients’ recent histories of colorectal screening tests, as determined by the history obtained at Visit 1.
We defined participation as (number of colonoscopies) / (number invited – number ineligible). Participation rates were similar in the two samples (P = 0.2; Box 2).
Among those eligible, 58.3% of the electoral roll sample underwent a colonoscopy, compared with 59.7% of the general practice sample. More than 90% of eligible subjects who did not have a screening colonoscopy declined the procedure at Visit 1; the remainder failed to attend. Both samples had similar responses to the first and second invitations, with 73.6% (170/231) of the colonoscopies performed requiring one invitation.
In total, 231 screening colonoscopies were performed. Of these, 229 were complete bowel examinations, resulting in a completion rate of 99%. Overall, 45% of participants had polyps identified and removed. Thirty-two per cent of participants had adenomatous polyps and 22.9% had hyperplastic polyps (Box 4). Advanced adenomas (10 mm or greater in size, or displaying high-grade dysplasia or prominent villous histology) were present in eight subjects (3.5%).
Participant satisfaction with colonoscopy screening was excellent, with 99% of the 219 subjects attending Visit 3 saying they were willing to have a colonoscopy in the future.
Expert groups uniformly recommend screening for colorectal cancer in people with average risk. Randomised clinical trials have demonstrated a reduction in CRC mortality of 15%–33% after screening by FOBT followed by colonoscopy in subjects testing positive.12-14 The evidence that screening by colonoscopy alone reduces CRC incidence is weaker, coming from cohort studies6,15 and a small randomised trial,16 although the level of prevention is 80%–90%. Colonoscopic screening is the preferred strategy of the American Cancer Society and the American College of Gastroenterology.5 The National Health and Medical Research Council recommends either biennial FOBT or 5-yearly flexible sigmoidoscopy for people at average risk.11
The efficacy of screening by either FOBT or colonoscopy is influenced by participation rates. To our knowledge, there are no results available from general population sample screening trials using colonoscopy; although large-scale studies have been published, subjects were referred or recruited from specialised populations.17-19 One study reported 45% participation among 7005 veterans, with 54% of those invited declining involvement.17 Initial participation in FOBT (performance of the first round of tests) ranges from 50% to 67%;14,20 participation rates decline thereafter, with 20%–50% of initial participants completing further examinations.21,22 Ongoing participation in FOBT screening outside clinical trials is typically less than 30%.23
In this study, we compared two population samples — from the ACT electoral roll and from GPs’ patient databases. The two recruitment approaches yielded similar participation rates, with 35% of the electoral roll sample participating and 40% participating from the general practice sample (note: these estimates include non-responders, who could not be assessed for eligibility, in the denominator). These rates are lower than initial participation in FOBT, but compare favourably with the 10-year compliance.20
We caution that the ACT has a relatively well-educated, high-income population that may respond more favourably to screening requests than the rest of Australia.24 However, evidence from the Australian population suggests that socioeconomic status does not affect participation in cancer screening.25,26
We encountered several limitations to GP-based recruitment. General practice databases were diverse, with half of the practices requiring manual review of records to select people for invitation. In addition, GPs had various archiving practices. Many potential subjects may have been excluded from the invitation round because their files had been archived. We could not determine if these patients had left the practice or were merely “inactive”. The labour of manual record sorting could be traded off against better eligibility. However, about 15% of records of general practice invitees did not contain histories of recent colorectal screening tests — a major source of ineligibility. Furthermore, recruitment through general practice databases does not ensure that the population will be proportionally represented, as our comparison to the 2001 matched ACT Census population estimates showed. Equity is a key principle of population screening,27 and these findings suggest that GP-based recruitment could reduce equity compared with a broader population approach.
The GPs we recruited to the study were enthusiastic. Others have reported that engaging GP participation can be an obstacle to screening studies.28,29
A weakness of the electoral roll approach was that more than a third of those invited were ineligible for colonoscopy screening (Box 2). On the other hand, a centralised invitation process allows control of the flow of people attending screening centres. During the study, this control was used to ensure resources were well managed.
The electoral roll sample showed that 19% of the population aged 55–74 years had a colonoscopy in the 10 years before recruitment. This provides a practical estimate to be used with modelled estimates in determining the costs of population CRC screening.
Our study was designed to test the observation from FOBT studies7,8 that an invitation for screening from a subject’s usual GP rather than a central organisation is more likely to lead to screening participation. Supporting this hypothesis, an Australian study also reported superiority of GP recruitment to an aspirin prevention study, with participation of 1 in 6 invitees (general practice) versus 1 in 17 (electoral roll).30 In contrast, we found that subjects invited by GPs were only slightly more likely to reply or attend the first visit for colonoscopic screening. Not surprisingly, those attending from GP invitations were more likely to meet eligibility criteria, but eligible invitees were no more likely to actually attend for colonoscopy. Therefore, the source of invitation played little role in the likelihood of a subject responding or attending. For consideration of design of large-scale population screening, the benefit of recruitment from general practices (superior eligibility) must be offset against the increase in recruitment work required, and in the likely uneven recruitment by region, and consequent effect on equity.
Colon cancer screening is highly cost-effective compared with other accepted healthcare interventions.31 The overall cost and personnel requirements of colonoscopic screening are sensitive to compliance rates and the prevalence of adenomatous polyps in the screened population.32 We have not presented an assessment of costs or personnel requirements, but our data may be useful for baseline assumptions.
This study provides useful data in relation to recruitment for large-scale CRC screening, particularly in the Australian setting. Electoral roll recruitment is simple, effective and yields better population coverage than general practice recruitment alone. General practice recruitment by itself has several limitations, and would need to be supported by other programs. Nevertheless, regardless of recruitment process, we found that it is possible to formally engage the community, GPs, gastroenterologists and healthcare organisations in population-based colorectal screening.
1 Exclusion criteria
Prior diagnosis of cancer, not including non-melanomatous skin cancer.
Colonoscopy, faecal occult blood test, sigmoidoscopy, barium enema or virtual colonoscopy within 10 years.
Recent onset of lower gastrointestinal tract symptoms (bleeding, change in bowel habit, abdominal pain, weight loss, bloating, anaemia) causing GP attendance in the previous 12 months.
Significant comorbidity (American Society of Anesthesiologists class III or greater.9
Previous colonic surgery.
Therapeutic anticoagulation.
Participation in a clinical trial in the previous 3 months.
A person unlikely to be compliant or unable to give informed consent.
3 Reasons for ineligibility among people classified as ineligible within the study’s samples
Reason for ineligibility |
Electoral roll |
General practice |
|||||||||||||
Previous colonoscopy |
98 (55%) |
42 (50%) |
|||||||||||||
Significant comorbidity |
12 (7%) |
1 (1%) |
|||||||||||||
Away from residence |
6 (3%) |
13 (16%) |
|||||||||||||
Diagnosed with cancer |
19 (11%) |
8 (10%) |
|||||||||||||
Barium enema |
7 (4%) |
9 (11%) |
|||||||||||||
Faecal occult blood test |
3 (2%) |
5 (6%) |
|||||||||||||
English language proficiency |
12 (7%) |
4 (5%) |
|||||||||||||
Other* |
21 (12%) |
2 (2%) |
|||||||||||||
Total |
178 (100%) |
84 (100%) |
|||||||||||||
* Other includes taking warfarin, recent surgery, anaemia, iron deficiency, GP already organised colonoscopy, and died. |
|||||||||||||||
4 Findings of adenomatous and hyperplastic polyps in 231 colonoscopies
Subjects in whom polyps were identified |
104 |
(45%) |
Subjects with advanced polyps |
8 |
(3.5%) |
Subjects with adenomatous polyps |
74 |
(32.0%) |
Subjects with hyperplastic polyps |
53 |
(22.9%) |
Received 8 April 2004, accepted 9 August 2004
- Mike Corbett1
- Sharon L Chambers2
- Bruce Shadbolt3
- Doug Taupin4
- Lybus C Hillman5
- 1 The Canberra Hospital, Woden, ACT.
- 2 Brindabella Specialist Centre, Garran, ACT.
This study was made possible by a grant from the Canberra Hospital Private Practice Trust Fund and a donation from Medical Specialists Associated. No Medicare charges were incurred in the study. We wish to thank participating GPs, particularly Drs Glynn Kelly, Peter Gibson, Bob Hain, Paul Jones, Tuck Meng Soo and Phillip Verghese, for their assistance with recruitment. The encouragement of the ACT Division of General Practice is gratefully acknowledged. We thank Dr Joanna Holt, Dr Mark Bassett and Susan Duthie RN for essential logistical support, including costing of colonoscopies; Ms Barbara Stuart Harris for performing Cancer Registry checks; endoscopists, anaesthetists, nursing and clerical staff at the participating endoscopy centres; and Dr Paul Dugdale and Professor Anne Gardner for helpful comments.
None identified.
- 1. Parkin DM, Whelm SL, Ferlay J, et al, editors. Cancer incidence in five continents. Vol VII. Lyon: IARC, 1997. (IARC Scientific Publication No. 143.)
- 2. Australian Institute of Health and Welfare and Australasian Association of Cancer Registries. Cancer survival in Australia, 2001. Part 1: National summary statistics. Canberra: Australian Institute of Health and Welfare, 2001. (Cancer Series No. 18; AIHW Catalogue No. CAN 13.)
- 3. Wingo PA, Ries LA, Giovino GA, et al. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst 1999; 91: 675-690.
- 4. Burt RW. Colon cancer screening. Gastroenterology 2000; 119: 837-853.
- 5. Ransohoff DF, Sandler RS. Clinical practice. Screening for colorectal cancer. N Engl J Med 2002; 346: 40-44.
- 6. Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993; 329: 1977-1981.
- 7. Hardcastle JD, Farrands PA, Balfour TW, et al. Controlled trial of faecal occult blood testing in the detection of colorectal cancer. Lancet 1983; 2: 1-4.
- 8. Cole SR, Young GP, Byrne D, et al. Participation in screening for colorectal cancer based on a faecal occult blood test is improved by endorsement by the primary care practitioner. J Med Screen 2002; 9: 147-152.
- 9. American Society of Anesthesiologists. ASA physical status classification system. Available at: www.asahq.org/clinical/physicalstatus.htm (accessed Jul 2004).
- 10. Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002; 97: 1296-1308.
- 11. National Health and Medical Research Council. Guidelines for the prevention, early detection and management of colorectal cancer. Canberra: NHMRC, 1999.
- 12. Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst 1999; 91: 434-437.
- 13. Hardcastle JD, Chamberlain JO, Robinson MHE, et al. Randomised controlled trial of faecal occult blood screening for colorectal cancer. Lancet 1996; 348: 1472-1477.
- 14. Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal occult blood test. Lancet 1996; 348: 1467-1471.
- 15. Jorgensen OD, Kronborg O, Fenger C. The Funen adenoma follow-up study. Incidence and death from colorectal carcinoma in an adenoma surveillance program. Scand J Gastroenterol 1993; 28: 869-874.
- 16. Thiss-Evensen E, Hoff GS, Sauar J, et al. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol 1999; 34: 414-420.
- 17. Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen average risk for CRC. N Engl J Med 2000; 343: 162-168.
- 18. Imperiale TF, Wagner DR, Lin CY, et al. Risk of proximal neoplasms in asymptomatic adults according to the distal colonoscopic findings. N Engl J Med 2000; 343: 169-174.
- 19. Scholefield JH. ABC of colorectal cancer: screening. BMJ 2000; 321: 1004-1006.
- 20. Jorgensen OD, Kronborg O, Fenger C. A randomized study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven diennial screening rounds. Gut 2002; 50: 29-32.
- 21. Winawer SJ, Flehinger BJ, Schottenfeld D, Miller DG. Screening for colorectal cancer with fecal occult blood testing and sigmoidoscopy. J Natl Cancer Inst 1993; 85: 1311-1318.
- 22. Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328: 1365-1371.
- 23. Vernon SW. Participation in colorectal cancer screening: a review. J Natl Cancer Inst 1997; 89: 1406-1422.
- 24. Australian Bureau of Statistics. Australian social trends. Canberra: ABS, 2004. (ABS Catalogue No. 4102.0.)
- 25. O’Byrne AM, Kavanagh AM, Ugoni A, Diver F. Predictors of non-attendance for second round mammography in an Australian mammographic screening programme. J Med Screen 2000; 7: 190-194.
- 26. Cole SR, Young GP, Esterman A, et al. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen 2003; 10: 117-122.
- 27. Canadian Strategy for Cancer Control: Screening Working Group. Population cancer screening in Canada: strategic priorities. 2002. Available at: www.cancercontrol.org/cscc/pdf/finalscreeningJan2002.PDF (accessed Jul 2004).
- 28. Clover KA, Redman S, Forbes JF, et al. Promotion of attendance for mammographic screening through general practice: a randomised trial of two strategies. Med J Aust 1992; 156: 91-94.
- 29. Cockburn J, Thomas RJ, McLaughlin SJ, Reading D. Acceptance of screening for colorectal cancer by flexible sigmoidoscopy. J Med Screen 1995; 2: 79-83.
- 30. Silagy CA, Campion K, McNeil JJ, et al. Comparison of recruitment strategies for a large-scale clinical trial in the elderly. J Clin Epidemiol 1991; 44: 1105-1114.
- 31. Drummond MF, O’Brien B, Stoddart GL, Torrance GW. Cost-effectiveness analysis. In: Methods for the economic evaluation of health care programmes. 2nd ed. New York: Oxford University Press, 1997: 96-138.
- 32. Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer. Ann Intern Med 2000; 133: 573-584.





Abstract
Objectives: To determine the response to colorectal cancer (CRC) screening by colonoscopy, through direct invitation or through invitation by general practitioners.
Design and setting: Two-way comparison of randomised population sampling versus cluster sampling of a representative general practice population in the Australian Capital Territory, May 2002 to January 2004.
Intervention: Invitation to screen, assessment for eligibility, interview, and colonoscopy.
Subjects: 881 subjects aged 55–74 years were invited to screen: 520 from the electoral roll (ER) sample and 361 from the general practice (GP) cluster sample.
Main outcome measures: Response rate, participation rate, and rate of adenomatous polyps in the screened group.
Results: Participation was similar in the ER arm (35.1%; 95% CI, 30.2%–40.3%) and the GP arm (40.1%; 95% CI, 29.2%–51.0%) after correcting for ineligibility, which was higher in the ER arm. Superior eligibility in the GP arm was offset by the labour of manual record review. Response rates after two invitations were similar for the two groups (ER arm: 78.8%; 95% CI, 75.1%–82.1%; GP arm: 81.7%; 95% CI, 73.8%–89.6%). Overall, 53.4% ineligibility arose from having a colonoscopy in the past 10 years (ER arm, 98/178; GP arm, 42/84). Of 231 colonoscopies performed, 229 were complete, with 32% of subjects screened having adenomatous polyps.
Conclusions: Colonoscopy-based CRC screening yields similar response and participation rates with either random population sampling or general practice cluster sampling, with population sampling through the electoral roll providing greater ease of recruitment.