Many health problems affecting older people are not routinely detected.1 Prevention-based assessment programs have been devised to help identify and treat these problems. However, reviews of the evidence for these programs have come to varying conclusions — that the assessments have positive effects,2,3 no effect,4 or clinically insignificant effects.5,6 These reviews also found differences between studies in content and duration of assessments and length of follow-up. The most recent review found that assessments are effective, but that this effectiveness depends on multiple follow-ups.2 It also found that the benefits are most pronounced for the “young-old” (under 78 years) and for those with a low risk of death.
To address the need for further trials of preventive health assessments for older Australians, the Australian Department of Veterans’ Affairs provided funding in 1996 for a randomised controlled Preventive Care Trial for older Australian veterans and war widows. The primary aim of the trial was to evaluate the effectiveness of assessments undertaken by existing healthcare services in improving quality of life, reducing hospital and nursing home admissions, and reducing deaths during 3 years of assessment and observation. We report here the results of the trial that relate to this aim.
Participants were randomly selected veterans or war widows who were receiving full entitlements from the Australian Department of Veterans’ Affairs, were aged 70 years or over, and were living in the community in randomly selected postal areas in 10 geographical regions (six in New South Wales and four in Queensland). Selection was stratified to allow equal representation of men and women and urban/rural residence, but over-representation of veterans aged 85 years and over. Selected veterans and widows received a letter from the Department of Veterans’ Affairs inviting them to participate in the study.
Participants who returned written consent to the study team were entered into the study database by the data manager and randomly allocated by computer using Statistical Applications Software (SAS)7 to one of four intervention groups or the control group. A minimisation method8 was applied to balance randomisation across areas, age groups (70–84 years, 85+ years) and sex. Randomisation was also designed to achieve an intervention to control ratio of 2 : 1. Trial participants and clinicians were not blinded to allocation.
The checklist was designed to identify problems common among older people that have significant negative impact on their lives and are amenable to intervention9 (Box 1). It included a specially developed home hazards assessment tool (HOME FAST).15 Most of the 113 items on the checklist relied on participants’ self-report. Medications were recorded from package labels identified in the home.
Measures were collected at baseline and at three annual follow-ups by computer-assisted telephone interview (CATI) or by post if telephone interview was not possible (eg, because of hearing impairment). Interviewers were not part of the intervention team and were blinded to participants’ trial status. Questions included the 36-item Medical Outcomes Study Short Form (SF-36)16 and general items assessing healthcare use, including admission to hospital in the previous year. The SF-36 converts to eight subscales and two summary scores — the Physical Health Component Summary and Mental Health Component Summary scores.16 In addition, all participants were cross-checked against the National Death Index after completion of all interviews in June 2001.
Statistical analyses were designed to test the primary hypothesis that veterans and war widows randomly allocated to receive at least annual assessments for 3 years will have higher scores on SF-36 quality-of-life measure, fewer acute hospital admissions, fewer nursing home admissions, and lower mortality rates than those randomly allocated to receive usual care. Secondary hypotheses related to the effect of different frequencies and intensities of intervention (annual or 6-monthly assessments, with and without reminders).
The study was designed to have 80% power to detect a 50% reduction in mortality and a 7% difference in the proportion reporting hospital admissions. The study was also designed to have sufficient participants to detect half a standard deviation difference in quality of life between any two intervention subsets.
Hospital and nursing home admissions and deaths were compared between intervention and control groups using χ2 analyses. The SF-36 Physical and Mental Health Components Summary scores were analysed for participants with a baseline score and data for at least one follow-up survey, using repeated-measures analysis, via the PROC MIXED procedure in SAS.17 Scores at the first, second and third year of follow-up were used, with adjustment for baseline SF-36 scores. An interaction between treatment group and follow-up time was modelled to obtain adjusted differences in the SF-36 scores.
Missing data were assumed to be missing at random. This assumption was examined by the approach of Wei and Stram,18 with missing status as the outcome, and intervention group and time as explanatory variables. An interaction between time and treatment group was fitted and did not show a systematic difference in the occurrence of missing data over time according to treatment group.
As the SF-36 subscale scores were highly non-normally distributed, they were divided into quartiles, and compared by ordinal logistic regression analysis, with adjustment for baseline scores and repeated measures.
A sensitivity analysis was undertaken to assess the degree to which the results were affected by missing SF-36 data arising because of death, admission to nursing home, or the patient being too ill to participate at that time point. These patients were assigned to the lowest quartile (worst outcome), and the ordinal logistic regression analysis was repeated.
The O’Brien–Fleming procedure19 was used to set levels of statistical significance to allow two interim analyses and a final analysis for the third year of follow-up. The null hypothesis was to be rejected if P values were less than 0.0006 at first follow-up, 0.015 at second follow-up, and less than 0.0475 at the final analysis.
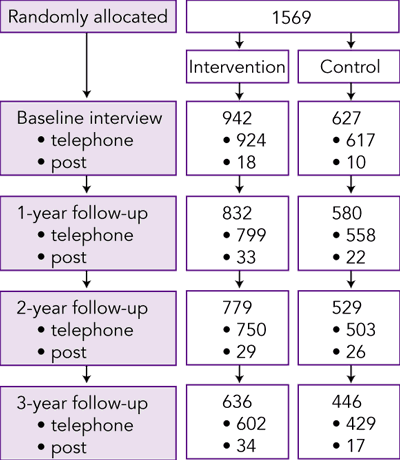
Participation in each stage of the trial is shown in Box 2. A total of 1569 veterans and war widows completed a baseline interview between December 1997 and April 1998 and were randomised to the intervention (n = 942) or control (n = 627) groups. Within the intervention group, 237 participants were allocated to Group 1, 237 to Group 2, 231 to Group 3, and 237 to Group 4.
At baseline, the control and intervention groups were similar in demographic characteristics and health indicators. Participants were also roughly representative of the general population in education level and occupational status when compared with the 1996 Australian census.20 Married women were greatly under-represented (3%), as “war widow” is the main entitlement category for women under Department of Veterans’ Affairs criteria.
Small numbers of participants completed the surveys by post at each time point. Their data were not included in the quality-of-life analyses because of the potential for measurement bias (patients tend to report lower SF-36 scores in postal surveys than in telephone interviews) and a much higher occurrence of missing data.
Participants allocated to the intervention groups were more likely to be recorded as having been permanently admitted to a nursing home than those in the control group: 30 (3.2%) in the intervention groups versus 7 (1.1%) in the control group (P < 0.01). There was no significant difference in the number of deaths: 123 (13.1%) in the intervention groups versus 66 (10.5%) in the control group (P = 0.13).
There was also no statistically significant difference between groups in the proportion admitted to hospital during the previous 12 months at any follow-up. At final follow-up, 41.1% in the intervention groups and 44.5% in the control group had been admitted to hospital in the previous year (P = 0.27).
Quality-of-life outcomes were compared for 1424 participants who completed the baseline telephone survey and at least one follow-up telephone survey. At 3-year follow-up, unadjusted mean Physical Components Summary scores were 47.6 for the intervention group versus 45.9 for the control group. Corresponding unadjusted mean Mental Component Summary scores were 50.6 and 49.4, respectively.
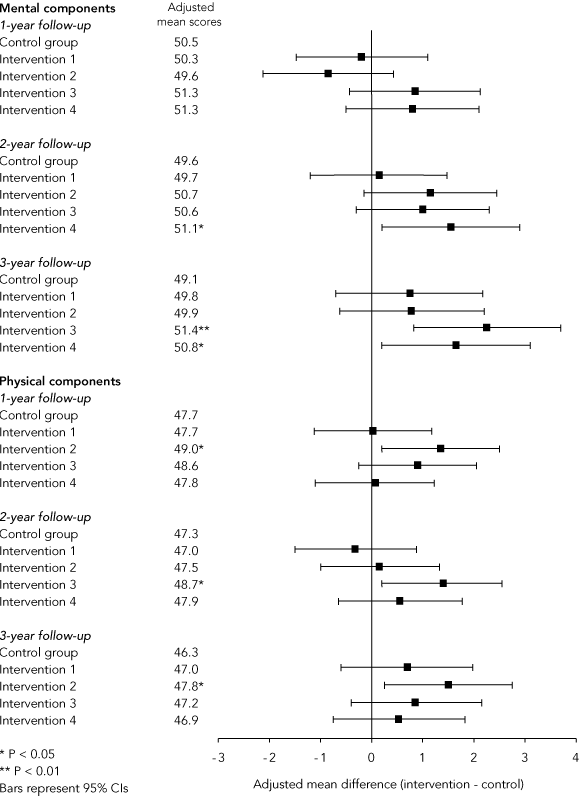
Repeated-measures analysis of summary scores showed that, after adjustment for baseline scores, the difference between the mean Physical Components Summary scores was statistically significant at final follow-up (P = 0.04). However, the estimate of effect was less than 1 unit (adjusted mean difference, 0.90; 95% CI, 0.05–1.76) on the standardised scale (scores are standardised to the Australian population in each age group, so that a score of 50 is “average” for that age). Similarly, the difference between adjusted mean Mental Components Summary scores was statistically significant at both 2-year (P = 0.03) and 3-year (P = 0.006) follow-ups, but adjusted mean differences were only 0.96 (95% CI, 0.07–1.85) and 1.36 (95% CI, 0.40–2.32), respectively.
The intervention groups were more likely to report higher scores for the SF-36 subscales of physical functioning, general health, vitality, and social functioning at 3-year follow-up (Box 3).
Box 4 shows results of the analysis of the effect of intensity and frequency of intervention. For Mental Component Summary scores, there was a consistent trend in favour of groups which received 6-monthly visits (Groups 3 and 4), with differences in adjusted mean scores of up to 2.3 points.
Sensitivity analysis based on the ordinal logistic regression modelling (where patients who were too ill to participate, had been admitted to a nursing home or had died were assigned to the worst outcome) did not show a statistically significant benefit of the intervention. This suggests that, for the worst-case scenario, the intervention does not make a significant impact on quality of life.
In this study, veterans and war widows who received health assessments and who remained in the study reported higher quality of life than control-group participants. However, the difference in quality-of-life scores was less than the difference associated with the presence of one chronic disease.21 There was a trend for more frequent assessments to result in greater differences in Mental Component Summary scores. There was no significant difference in the probability of hospital admission or death over the study period. A significantly higher proportion of the intervention group were admitted to nursing homes compared with the control group (P < 0.01).
The strengths of this study are the large sample size, the duration of follow-up and the ability to undertake subset analyses. The study may suffer from loss to follow-up, and the increase in admission to nursing homes among people in the intervention group may have been a factor in the observed differences in quality of life at the end of the study. Sensitivity analyses to substitute for missing outcomes from these participants (and others who died or were too ill) suggest that, in the worst-case scenario, the effect on quality of life may be lost. Another limitation is the extent to which these findings apply to all older people, as participants were veterans and war widows entitled to veterans’ benefits. It could be argued that entitled veterans already have greater access to services, potentially reducing the impact of health assessments (due to lower baseline need), or, alternatively, increasing their impact (due to the greater ability to intervene).
This study’s findings are consistent with those of many other trials of geriatric assessment, which found improvements in perceived health of veterans and war widows, but no reductions in mortality. Of 21 trials reviewed by Byles et al, only four reported reductions in the number of deaths (or improved survival) among the intervention group.5 Other studies may not have observed differences in mortality because of small sample sizes or shorter periods of observation. A meta-analysis of seven randomised controlled trials of “home assessment services”, designed to overcome the problem of low power in smaller studies, concluded that such interventions could reduce mortality, hospital admission and institutionalisation.22
In our study, there was an increase in admissions to nursing homes in the intervention groups. This finding is at odds with what may be seen as a desirable outcome of assessment and is inconsistent with overseas studies. However, in a context where many people are unclear about how to access nursing homes,23 it is logical that increasing assessment and advice resulted in a greater proportion of people being admitted to residential care.
The outcomes of health assessments must also be measured relative to the cost of the intervention. We estimated that the average cost of each visit was $116 (including travel and administration time). Without an observed reduction in deaths or hospitalisations, the small benefits in quality of life may not be considered cost-effective. Targeting specific subgroups might increase the cost-effectiveness of health assessments and requires further consideration. Other trials of geriatric assessment report different results for specific subgroups. For example, two trials have shown decreased dependency for activities of daily living and improved self-rated health and performance of household activities in participants with poorer health at baseline.24,25 The question whether health assessments benefit only “healthy” elderly or the “young” elderly has also been raised, with variable results.24-26
The effectiveness of preventive assessments is still debated.3,26 Our study found that a series of structured preventive assessments was associated with small improvements in quality of life, but no significant difference in deaths or hospital admission, and a significant increase in nursing home admissions. Further, the differences in quality of life gained significance only during the final years of the intervention, suggesting that a long lead-time is required to make a significant impact on health outcomes. Also, the differences in mental health were greater for groups receiving 6-monthly visits than for those receiving annual visits, suggesting that visits may need to be frequent to achieve this outcome.
Given these findings, the cost-effectiveness of health assessments for all older people should be carefully considered. Importantly, the approach to assessment evaluated in this study has major overlap with the Medicare Benefits Schedule Enhanced Primary Care Health Assessment items currently used extensively in Australian general practice. The study therefore provides valuable data on health policy for the Australian community, health providers and government.
1 Areas of assessment*
Use of hearing aids |
Self-rated health |
||||||||||||||
* The full checklist for assessment is available on request from the Department of Veterans’ Affairs, National Office, Canberra, ACT (www.dva.gov.au). |
|||||||||||||||
2 Participation in each stage of the trial
Reasons for dropout |
Intervention |
Control |
|||||||||||||
Died* |
115* |
61* |
|||||||||||||
Admitted to nursing home/hostel† |
29† |
7 |
|||||||||||||
Too ill |
26 |
6 |
|||||||||||||
Moved |
23 |
11 |
|||||||||||||
Refused |
33 |
22 |
|||||||||||||
No response |
80 |
74 |
|||||||||||||
Total |
306 |
181 |
|||||||||||||
* Analysis of deaths also included 11 people who dropped out because of nursing home admission but later died within the study period (7, intervention; 4, control), as well as two people who completed the 3-year interview but also died within the study period (1, intervention ; 1, control). † Analysis of nursing home admissions also included one person from the intervention group who completed the 3-year interview but was admitted to a nursing home immediately after. |
|||||||||||||||
3 Ordinal logistic regression modelling for the subscales of the Medical Outcomes Study Short Form (SF-36), showing median scores*
|
Baseline |
1-year follow-up |
2-year follow-up |
3-year follow-up |
|||||||||||
SF-36 subscale |
Inter- vention |
Control |
Inter- vention |
Control |
Odds ratio† |
P |
Inter- vention |
Control |
Odds ratio† |
P |
Inter- vention |
Control |
Odds ratio† |
P |
|
Physical functioning |
60 |
60 |
60 |
60 |
1.11 |
0.33 |
60 |
55 |
1.24 |
0.06 |
60 |
55 |
1.38 |
0.009 |
|
Role limitation physical |
50 |
50 |
50 |
50 |
1.20 |
0.08 |
50 |
25 |
1.12 |
0.30 |
25 |
25 |
1.17 |
0.18 |
|
Bodily pain |
62 |
61 |
62 |
62 |
0.99 |
0.99 |
62 |
61 |
1.07 |
0.51 |
62 |
52 |
1.22 |
0.09 |
|
General health |
60 |
57 |
65 |
62 |
1.18 |
0.13 |
62 |
60 |
1.04 |
0.73 |
62 |
57 |
1.48 |
0.001 |
|
Vitality |
55 |
55 |
50 |
50 |
1.23 |
0.05 |
55 |
50 |
1.23 |
0.06 |
55 |
50 |
1.53 |
0.001 |
|
Social functioning |
88 |
88 |
88 |
88 |
1.00 |
0.99 |
88 |
75 |
1.06 |
0.58 |
88 |
75 |
1.47 |
0.001 |
|
Role limitation emotional |
100 |
100 |
100 |
100 |
1.11 |
0.43 |
100 |
100 |
1.35 |
0.02 |
100 |
100 |
1.20 |
0.17 |
|
Mental health |
84 |
84 |
88 |
84 |
1.17 |
0.14 |
84 |
84 |
1.10 |
0.38 |
84 |
84 |
1.24 |
0.07 |
|
* Each subscale has a possible range of 0–100, with the population mean and impact of disease varying between subscales. For example, the Australian Bureau of Statistics National Health Survey found mean scores for physical functioning were 89.4 (no disease), 83.2 (one disease) and 73.4 (two diseases).21 † Odds ratio for intervention group versus control group. |
|||||||||||||||
Received 10 September 2003, accepted 17 June 2004
- Julie E Byles1
- Meredith Tavener2
- Nick H Higginbotham3
- Lyn Francis4
- John E Marley5
- Rachel L O’Connell6
- Balakrishnan R Nair7
- Brendan G Goodger8
- Claire L Jackson9
- Mary E McKernon10
- Richard F Heller11
- Jonathan Newbury12
- 1 Faculty of Health, University of Newcastle, Newcastle, NSW.
- 2 Hunter Area Health Service and John Hunter Hospital, Newcastle, NSW.
- 3 NHMRC Clinical Tr ials Centre, University of Sydney, Sydney, NSW.
- 4 Mater Centre for Integrated Health Care and General Practice, University of Queensland, Brisbane, QLD.
- 5 School of Epidemiology and Health Sciences, University of Manchester, Manchester, UK.
- 6 Department of General Practice, Adelaide University, Adelaide, SA.
The Preventive Care Trial was initiated and funded by the Australian Department of Veterans’ Affairs. We thank Professor John McCallum, scientific advisor for the study. We also thank Associate Professor Cate D’Este, Dr Patrick Fitzgerald and Associate Professor Lynette Lim for statistical advice; and Dr Lynette Mackenzie for developing the HOME FAST tool used in the home-visit checklist.
None identified.
- 1. Tulloch AJ, Moore VA. Randomized controlled trial of geriatric screening and surveillance in general practice. J R Coll Gen Pract 1979; 29: 733-742.
- 2. Stuck AQ, Egger M, Hammer A, et al. Home visits to prevent nursing home admission and functional decline in elderly people. JAMA 2002; 287: 1022-1028.
- 3. Van Haastregt JCM, Diederiks JPM, van Rossum E, et al. Effects of preventive home visits to elderly people living in the community: systematic review. BMJ 2000; 320: 754-758.
- 4. Cole MG. Impact of geriatric home screening services on mental state: a systematic review. Int Psychogeriatr 1998; 10: 97-102.
- 5. Byles JE. A thorough going over: evidence for health assessments for older persons. ANZ J Public Health 2000; 24: 117-123.
- 6. Elkan R, Kendrick D, Dewey M, et al. Effectiveness of home based support for older people: systematic review and meta-analysis. BMJ 2001; 323: 719.
- 7. SAS Institute. Statistical applications software, release 8. Cary, NC: SAS Institute, 1999.
- 8. White S, Freedman L. Allocation of patients to treatment groups in a controlled clinical study. Br J Cancer 1978; 37: 103-115.
- 9. Byles JE, Tavener M, Fitzgerald P, et al. A checklist for comprehensive health assessment for the over 70’s. Austr J Ageing 2002; 21: 14-20.
- 10. Lipski PS. Australian Nutrition Screening Initiative. Aust J Ageing 1996; 5: 14-17.
- 11. Stewart AL, Kamberg CJ. Physical functioning measures. In: Stewart AL, Ware JE Jr (eds). Measuring functioning and well being: the Medical Outcomes Study approach. Durham, NC: Duke University Press, 1992: 86-101.
- 12. Koenig HG. An abbreviated Mini-Mental State Exam for medically ill older adults. J Am Geriatr Soc 1996; 44: 215-216.
- 13. Koenig HG, Westlund R, George LK, et al. Abbreviating the Duke Social Support Index for use in chronically ill elderly individuals. Psychosomatics 1993; 34: 61-69.
- 14. Byles JE, Higginbotham N, Goodger B, et al. Depression among older Australian veterans and war widows. Int J Behav Med 2000; 7: 256-270.
- 15. Mackenzie L, Byles J, Higginbotham N. Designing the Home Falls and Accidents Screening Tool (HOME FAST): selecting the items. Br J Occup Ther 2000; 63: 260-269.
- 16. Ware JE, Sherbourne CD. The MOS 36-item Short Form Health Survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473-483.
- 17. Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute, 1996.
- 18. Wei LJ, Stram DO. Analysing repeated measurements with possibly missing observations by modelling marginal distributions. Stats In Med 1988; 7: 139-148.
- 19. O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics 1979; 35: 549-556.
- 20. Australian Bureau of Statistics. Census of population and housing. Selected family and labour force characteristics. Canberra: ABS, 1996. (Catalogue No. 2017.0.)
- 21. Australian Bureau of Statistics. National Health Survey. SF-36 population norms, Australia. Canberra: ABS, 1995. (Catalogue No. 4399.0.)
- 22. Stuck AE, Siu AL, Wieland GD, et al. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet 1993; 342: 1032-1036.
- 23. Fox A, Sims S, Farish S, et al. A specialised advisory service for older people and those caring for them. Aust J Ageing 1995; 14: 68-70.
- 24. Bula CJ, Berod AC, Stuck AE, et al. Effectiveness of preventive in-home geriatric assessment in well functioning, community-dwelling older people: secondary analysis of a randomized trial. J Am Geriatr Soc 1999; 47: 389-395.
- 25. Van Rossum E, Frederiks CMA, Philipsen H, et al. Effects of preventive home visits to elderly people. BMJ 1993; 307: 27-32.
- 26. Newbury J, Marley J, Beilby J. A randomised controlled trial of the outcome of health assessment of people aged 75 years and over. Med J Aust 2001; 175: 104-107.





Abstract
Objective: To assess the effect of home-based health assessments for older Australians on health-related quality of life, hospital and nursing home admissions, and death.
Design: Randomised controlled trial of the effect of health assessments over 3 years.
Participants and setting: 1569 community-living veterans and war widows receiving full benefits from the Department of Veterans’ Affairs and aged 70 years or over were randomly selected in 1997 from 10 regions of New South Wales and Queensland and randomly allocated to receive either usual care (n = 627) or health assessments (n = 942).
Intervention: Annual or 6-monthly home-based health assessments by health professionals, with telephone follow-up, and written report to a nominated general practitioner.
Main outcome measures: Differences in health-related quality of life, admission to hospital and nursing home, and death over 3 years of follow-up.
Results: 3-year follow-up interviews were conducted for 1031 participants. Intervention-group participants who remained in the study reported higher quality of life than control-group participants (difference in Physical Component Summary score, 0.90; 95% CI, 0.05–1.76; difference in Mental Component Summary score, 1.36; 95% CI, 0.40–2.32). There was no significant difference in the probability of hospital admission or death between intervention and control groups over the study period. Significantly more participants in the intervention group were admitted to nursing homes compared with the control group (30 v 7; P < 0.01).
Conclusions: Health assessments for older people may have small positive effects on quality of life for those who remain resident in the community, but do not prevent deaths. Assessments may increase the probability of nursing-home placement.