Type 2 diabetes is a progressive disease. Despite ongoing lifestyle interventions and dose increments of sulfonylurea, metformin or insulin, the disease remains suboptimally controlled in about 50% of patients 6 years after being diagnosed.1 This relates, in part, to the observation that neither sulfonylurea nor metformin therapy arrests the progressive decline in b-cell function seen in type 2 diabetes.1,2
The thiazolidinediones (TZDs) pioglitazone and rosiglitazone are novel insulin-sensitising antidiabetic agents that bind with high affinity to the peroxisome proliferator-activated receptor (PPAR-γ) .3 Studies in animals4 and humans5 suggest that TZDs may help preserve b-cell function and thereby delay progression of type 2 diabetes.
A number of clinical trials have reported use of TZD as monotherapy6-8 or in combination with a sulfonylurea, metformin or insulin,9-11 and have shown significant reductions in the level of glycohaemoglobin (HbA1c) compared with placebo. However, there are few reports of effectiveness and side effects of TZDs in routine clinical use, and few studies comparing rosiglitazone and pioglitazone.12-15 We have therefore conducted a review of TZDs in clinic outpatients with type 2 diabetes whose disease was not optimally controlled with standard therapy.
A total of 203 patients who attended the diabetes clinic at Royal Melbourne Hospital were prescribed TZDs for two months or more between 1 May 2000 and 31 October 2002 (Box 1). Before treatment there were no significant differences in age, sex, weight, HbA1c level or lipid profiles between the patients treated with pioglitazone or rosiglitazone. The mean duration of diabetes was significantly longer in the pioglitazone group than the rosiglitazone group. Half the patients in each group (50%) were receiving hypolipidaemic agents.
Both pioglitazone and rosiglitazone were associated with a significant reduction in HbA1c level of around 1% at 6 months (Box 2a). Baseline diabetes treatment did not significantly influence the effect of TZD on HbA1c level.
In a smaller population of patients for whom HbA1c level was recorded at baseline, 6 and 12 months, a similar 1% reduction was seen and sustained (Box 2b).
In patients for whom data were available, statistically significant increases in total cholesterol and triglyceride levels were associated with rosiglitazone, but not pioglitazone, treatment (Box 2c and 2d).
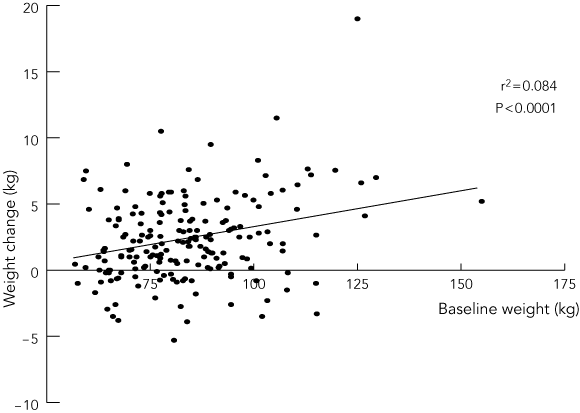
Repeat weight measurements during the first 6 months of TZD treatment were available for 192 patients. Just over half of these experienced weight gain of more than 2 kg (Box 3). The mean gain was 2.3 kg (range, –5.0 to 19 kg) in the pioglitazone group and 2.9 kg (range, –5.0 to 11.5 kg) in the rosiglitazone group (P = 0.95). Baseline treatment for diabetes (OHA v insulin v OHA + insulin) did not correlate with degree of weight gain. However, there was significant correlation between baseline weight and weight gain at six months (Box 4).
We report the effects of TZD therapy in routine Australian clinical practice. We acknowledge that the study’s retrospective, non-randomised design is a limitation.
Our study population, whose average HbA1c level was clearly elevated at 9.6%, was representative of the Australian population of people with type 2 diabetes for whom TZD therapy might be considered. While our prescribing patterns did not follow current Pharmaceutical Benefits Scheme (PBS) authority guidelines (eg, rosiglitazone was combined with insulin; TZD was used with more than one other OHA), we believe most of our patients would have met the criteria for the authority guidelines, which were developed after we started to prescribe TZDs.
Consistent with other studies,6-11,14,16,17 we have shown TZD treatment is associated with a clinically significant reduction in HbA1c level of about 1%. It could be argued that this reduction is caused by other factors, such as increased patient compliance with diet, exercise or medication. However, our patients were not offered additional diabetes clinical care or education in conjunction with TZD treatment. Furthermore, in general, these patients were a group who, over a number of years, had had various interventions in an attempt to improve glycaemia, but remained suboptimally controlled. We therefore feel the observed reductions in HbA1c level are real effects of TZD treatment.
We observed a significant increase in total cholesterol and triglyceride levels in patients receiving rosiglitazone, but not in those receiving pioglitazone. Previous prospective17 and retrospective14,15 studies have shown that rosiglitazone increases total cholesterol level, but may either increase6,17 or mildly decrease10,14,15 triglyceride levels. Pioglitazone has been shown to decrease both total cholesterol and triglyceride levels,11,14,15 a finding we did not confirm. Our results must be interpreted with caution, as patient numbers were small and we did not control for the use of lipid-lowering therapy. Given the important impact of dyslipidaemia on cardiovascular risk in type 2 diabetes,18 further study of the clinical significance of TZD-induced dyslipidaemia is highly desirable.
Our study also highlights the high rate of side effects associated with TZD in routine Australian practice. Hypoglycaemia was increased in about 15% of patients being treated with TZD, all of whom were also treated with either sulfonylurea and/or insulin. This rate of hypoglycaemia is much higher than previously reported in controlled trials.7,10,11 We strongly recommend that patients with diabetes who are treated with sulfonylurea and/or insulin are counselled about the increased risk of hypoglycaemia when starting TZD therapy.
Our results also confirm that weight gain is a significant side effect of TZD treatment.12 This was highly correlated with baseline body weight. Given the highly distressing nature of this side effect, it is surprising that weight gain only prompted cessation of therapy in a small proportion (2.5%) of our patients. Further study into its causes and possible prevention is needed.
The incidence of peripheral oedema of about 27% in our study was somewhat higher than the 3%–16% reported in other studies.6-13,16,17,19 This discrepancy may have resulted from our broader inclusion criteria or concurrent use of non-steroidal anti-inflammatory drugs and calcium-channel blockers (prescribed to 24% of all patients who experienced oedema in our study). Similarly, the 2.5% incidence of pulmonary oedema in our study was much higher than in other studies. We strongly recommend that TZD be used with great caution in patients with congestive cardiac failure and that clinical evidence of volume overload be sought when following any patient who commences TZD therapy.
Our study identified only two out of 203 patients with unexplained elevation of liver enzyme levels and no patients with permanent liver damage. These findings are insufficient to conclude that TZD treatment carries no hepatic risk. Until more safety data are available, we recommend 2-monthly liver function testing in all patients treated with TZD, as per Australian Therapeutic Goods Administration guidelines.12
Our observed rate of withdrawal from TZD treatment at 12 months (20%) was also at odds with results of controlled trials of TZD therapy.6-13 This may have resulted from inclusion of older patients or patients with significant comorbid disease, such as heart failure.
In summary, treatment of type 2 diabetes with pioglitazone or rosiglitazone in a hospital outpatient setting was associated with improved glucose control in most patients. The treatment was not without significant risk of side-effects, including hypoglycaemia, weight gain and oedema, which in turn led to a high cessation rate at 12 months. Careful patient selection and follow-up and realistic treatment goals are necessary for effective use of these novel agents.
1 Patient characteristics at baseline
Characteristic |
Pioglitazone |
Rosiglitazone |
Total |
P* |
|||||||||||
Number of patients |
107 |
96 |
203 |
|
|||||||||||
Age (years) |
|
|
|
|
|||||||||||
Mean (SD) |
64.4 (10.7) |
64.6 (10.3) |
64.5 (10.5) |
0.84 |
|||||||||||
Range |
36–86 |
41–82 |
36–86 |
|
|||||||||||
Sex (ratio, female : male) |
56:51 |
53:43 |
109:94 |
|
|||||||||||
Duration of diabetes mellitus (years) |
|
||||||||||||||
Mean (SD) |
17.0 (7.6) |
14.5 (17.3) |
15.8 (7.6) |
0.02 |
|||||||||||
Range |
3–36 |
4–33 |
3–36 |
|
|||||||||||
Baseline weight (kg) |
|
|
|
|
|||||||||||
Mean (SD) |
84.3 (14.1) |
82.3 (17.9) |
83.4 (16.0) |
0.38 |
|||||||||||
Range |
59.5–125.9 |
54.0–155.0 |
54.0–155.0 |
|
|||||||||||
Baseline HbA1c level (%) |
|
|
|
|
|||||||||||
Mean (SD) |
9.5 (1.6) |
9.6 (1.5) |
9.6 (1.6) |
0.48 |
|||||||||||
Range |
6.2–15.0 |
6.3–15.3 |
6.2–15.3 |
|
|||||||||||
Prior treatment (No. of patients [% of total]) |
|
||||||||||||||
Oral hypoglycaemic agent |
24 (22%) |
49 (51%) |
73 (36%) |
|
|||||||||||
Monotherapy |
3 |
10 |
13 |
|
|||||||||||
Double-agent therapy |
14 |
29 |
43 |
|
|||||||||||
Triple-agent therapy |
7 |
10 |
17 |
|
|||||||||||
Insulin monotherapy |
19 (18%) |
19 (20%) |
38 (19%) |
|
|||||||||||
Combination therapy† |
64 (60%) |
28 (29%) |
92 (45%) |
|
|||||||||||
Baseline insulin requirement |
|
|
|
|
|||||||||||
Mean units/day (SD) |
78 (48) |
76 (37) |
77 (44) |
|
|||||||||||
Range (units/day) |
8–216 |
14–150 |
8–216 |
|
|||||||||||
Mean units/kg/day (SD) |
0.91 (0.54) |
0.90 (0.49) |
0.90 (0.49) |
0.70 |
|||||||||||
Mean (SD) baseline lipid level (mmol/L) |
|
||||||||||||||
Total cholesterol |
4.95 (1.13) |
4.99 (0.76) |
4.96 (0.98) |
0.83 |
|||||||||||
Triglycerides |
2.72 (2.98) |
2.65 (2.14) |
2.69 (2.65) |
0.88 |
|||||||||||
* Pioglitazone versus rosiglitazone. † Oral hypoglycaemic agent plus insulin. SD = standard deviation. HbA1c = glycohaemoglobin. |
|||||||||||||||
2 Effect of thiazolidinediones on glycohaemoglobin (HbA1c) and lipid levels
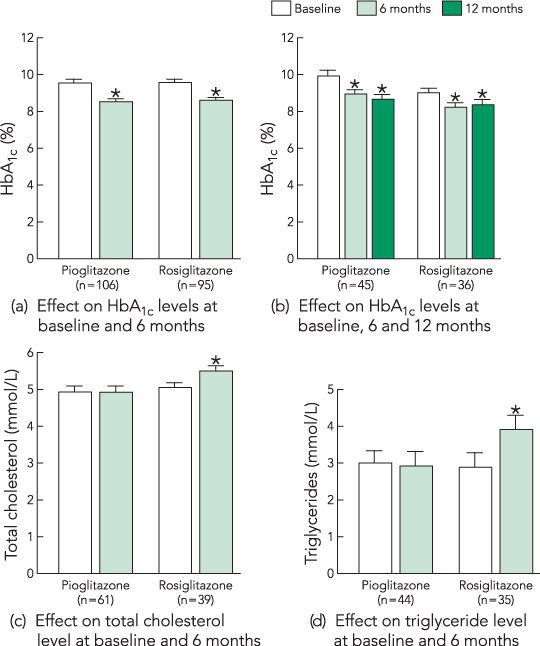
Data are shown as mean values and standard errors of the mean. * Significantly different from baseline values, calculated by the paired t test.
3 Effect of thiazolidinediones on weight gain at 6 months
|
Weight gain |
||||||||||||||
|
Nil |
Mild |
Moderate |
Severe |
|||||||||||
Combined (n = 192) |
96 (50%) |
61 (32%) |
32 (16%) |
3 (2%) |
|||||||||||
Thiazolidinediones |
|
|
|
|
|||||||||||
Pioglitazone (n = 103) |
54 (52%) |
32 (31%) |
15 (15%) |
2 (2%) |
|||||||||||
Rosiglitazone (n = 89) |
42 (47%) |
29 (33%) |
17 (19%) |
1 (1%) |
|||||||||||
Received 21 May 2004, accepted 23 September 2004
- 1. UK prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. UK Prospective Diabetes Study Group Diabetes 1995; 44: 1249-1258.
- 2. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA 1999; 281: 2005-2012.
- 3. Olefsky JM. Treatment of insulin resistance with peroxisome proliferator-activated receptor gamma agonists. J Clin Invest 2000; 106: 467-472.
- 4. Finegood DT, McArthur MD, Kojwang D, et al. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes 2001; 50: 1021-1029.
- 5. Buchanan TA. Pancreatic beta-cell loss and preservation in type 2 diabetes. Clin Ther 2003; 25 Suppl B: B32-46.
- 6. Phillips LS, Grunberger G, Miller E, et al. Rosiglitazone Clinical Trials Study Group. Once- and twice-daily dosing with rosiglitazone improves glycemic control in patients with type 2 diabetes. Diabetes Care 2001; 24: 308-315.
- 7. Aronoff S, Rosenblatt S, Braithwaite S, et al. Pioglitazone hydrochloride monotherapy improves glycemic control in the treatment of patients with type 2 diabetes: a 6-month randomized placebo-controlled dose-response study. The Pioglitazone 001 Study Group. Diabetes Care 2000; 23: 1605-1611.
- 8. Lebovitz HE, Dole JF, Patwardhan R, et al. Rosiglitazone Clinical Trials Study Group. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab 2001; 86: 280-288.
- 9. Raskin P, Rendell M, Riddle MC, et al. Rosiglitazone Clinical Trials Study Group. A randomized trial of rosiglitazone therapy in patients with inadequately controlled insulin-treated type 2 diabetes. Diabetes Care 2001; 24: 1226-1232.
- 10. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a randomized controlled trial. JAMA 2000; 283: 1695-1702.
- 11. Kipnes MS, Krosnick A, Rendell MS, et al. Pioglitazone hydrochloride in combination with sulfonylurea therapy improves glycemic control in patients with type 2 diabetes mellitus: a randomized, placebo-controlled study. Am J Med 2001; 111: 10-17.
- 12. O’Moore-Sullivan TM, Prins JB. Thiazolidinediones and type 2 diabetes: new drugs for an old disease. Med J Aust 2002; 176: 381-386. <MJA full text>
- 13. Khan MA, St Peter JV, Xue JL. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 2002; 25: 708-711.
- 14. Olansky L, Marchetti A, Lau H. Multicenter retrospective assessment of thiazolidinedione monotherapy and combination therapy in patients with type 2 diabetes: comparative subgroup analyses of glycemic control and blood lipid levels. Clin Ther 2003; 25 Suppl B: B64-B80.
- 15. Boyle PJ, King AB, Olansky L, et al. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Clin Ther 2002; 24: 378-396.
- 16. Einhorn D, Rendell M, Rosenzweig J, et al. Pioglitazone hydrochloride in combination with metformin in the treatment of type 2 diabetes mellitus: a randomized, placebo-controlled study. The Pioglitazone 027 Study Group. Clin Ther 2000; 22: 1395-1409.
- 17. Dailey GE 3rd, Noor MA, Park JS, et al. Glycemic control with glyburide/metformin tablets in combination with rosiglitazone in patients with type 2 diabetes: a randomized, double-blind trial. Am J Med 2004; 116: 223-229.
- 18. Turner RC, Millns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998; 316: 823-828.
- 19. Hollenberg NK. Considerations for management of fluid dynamic issues associated with thiazolidinediones. Am J Med 2003; 115 Suppl 8A: 111S-115S.





Abstract
Objective: To assess effectiveness and side effects of thiazolidinediones (TZDs) as adjunctive therapy in suboptimally controlled patients with type 2 diabetes.
Design and setting: Review of a prospectively recorded database at the Royal Melbourne Hospital diabetes clinic.
Participants: 203 patients with type 2 diabetes who received pioglitazone or rosiglitazone between 1 May 2000 and 31 October 2002.
Outcome measures: Response in glycohaemoglobin (HbA1c) level, lipid profile changes and side effects, including hypoglycaemia, weight gain, oedema and precipitation of cardiac failure.
Results: Both pioglitazone and rosiglitazone improved glycaemic control, with a reduction in the HbA1c level of 1.02% (range, 0.85%–1.19%) and 0.96% (range, 0.81%–1.11%), respectively, in the first 6 months of therapy. Rosiglitazone was associated with a 0.45 mmol (range, 0.31–0.59 mmol) increase in cholesterol level and 0.99 mmol (range, 0.60–1.38 mmol) increase in triglyceride level, while pioglitazone was associated with insignificant declines in cholesterol and triglyceride levels. There was reduced requirement for insulin, but not for oral hypoglycaemic agent (OHA), in most patients who used these agents. Pioglitazone and rosiglitazone were associated with increased rates of hypoglycaemia (17% and 11% of patients, respectively), significant weight gain (48% and 58%) and oedema (33% and 21%). There were four cases of acute left ventricular failure and two cases of reversible liver dysfunction in patients treated with TZDs.
Conclusions: Adding pioglitazone or rosiglitazone therapy to OHA or insulin in patients with type 2 diabetes significantly improved glycaemic control. However, the use of these drugs in routine clinical practice was associated with more frequent adverse events than previously reported in clinical trials.