The essential trace element selenium (Se) is a component of selenoproteins, some of which have important enzymic functions. These include the glutathione peroxidases, which reduce hydrogen peroxide and harmful lipid and phospholipid hydroperoxides, and the iodothyronine deiodinases, which catalyse the production of active thyroid hormone, tri-iodothyronine, from thyroxine.1-4
While the selenium-deficiency conditions Keshan disease (a cardiomyopathy) and Kashin–Beck disease (a debilitating osteoarthropathy) have long been recognised in China and Siberia1,2 (although they are unknown in Australia), mounting evidence suggests that less overt deficiency may be associated with reduced immunocompetence; increased virulence of RNA viruses; increased rate of progression to AIDS among HIV-positive individuals;1,3,5 increased rate of progression to cirrhosis or liver cancer among individuals with hepatitis B or C; increased risk of a range of cancers and other conditions that involve high levels of oxidative stress; impaired thyroid function; depression, anxiety and accelerated cognitive decline; and reduced fertility.1,3,4,6 Furthermore, supranutritional Se intake may provide additional protection against certain diseases, including some cancers.1-4,6
Most Se ingested by animals and humans comes from the soil, through plants. Levels of Se available in soils are highly variable globally. Areas that are notably low in Se include parts of China, Siberia, central Africa, eastern Europe, and New Zealand.2 Although large areas have not yet been mapped for Se, it is apparent that many people have too little Se to support maximum selenoenzyme expression.1-3
Human Se status is sensitive to changes in the food supply, in particular wheat,1 which is estimated to supply about half of the Se intake of most adult Australians.7 Of concern is a trend towards a reduction of Se in the food chain in certain regions, possibly because of fossil fuel burning (with release of sulfur, an Se antagonist), acid rain, soil acidification and use of high-sulfur fertilisers.8,9
Concentrations of Se in plasma and whole blood provide useful indicators of human Se intake and status. The concentration of Se in plasma is about 80% of that in whole blood.2 Box 1 shows reference values for plasma Se concentration in specified circumstances.
We undertook this study to compare plasma Se levels in a sample of healthy South Australian adults in 2002 with current global levels, and to compare 2002 South Australian plasma and whole blood Se levels with those found in previous surveys in South Australia, since 1977, to assess time trends, and the relationships of whole blood and plasma Se concentrations to sex and age.
2002 survey: 288 samples of whole blood were obtained from blood donated at the Australian Red Cross Blood Service, Adelaide, from 26–28 June 2002. Donor approval was obtained, and the age and sex of each donor recorded. This group could be considered a healthy sample of the population, having been screened by the Red Cross for haemoglobin levels, hepatitis B and C, and HIV.
Earlier surveys: Human blood Se data for 546 people determined by one of us (G J J) in 1977 and 1988 were included in the study. These included unpublished data from Australian Red Cross Blood Service donors and published studies of employees of the Institute of Medical and Veterinary Science13 and Kangaroo Island residents.14 The surveys were conducted in 1977 (117 participants), 1979 (30 participants), 1987 (96 and 103 participants), and 1988 (200 participants). All of these groups comprised apparently healthy adults, with mean age and age range similar to the 2002 sample. When the data for all the surveys were combined, the total sample size was 834 (445 men, 389 women), with a mean age of 42 years (range, 17–71 years).
2002 survey: Whole blood samples were stored at 4°C during the collection period, and whole blood from a subsample of 28 participants, and plasma samples from all 288 participants (obtained by centrifugation), were stored at –20°C for one week before analysis. Whole blood and plasma samples were digested with a nitric/perchloric acid mixture and finished with hydrochloric acid, then treated with sodium borohydride before analysis by hydride inductively-coupled plasma optical emission spectrometry (ICPOES), based on an established method.15 Variability between analytical runs and quality controls (serum and whole blood standards) was within acceptable limits.
Earlier surveys: In the surveys before 2002, Se concentrations were analysed by a fluorimetric method. 16,17 For each survey the analyses were conducted within one month of sample collection. Samples were stored at 4°C for up to a week, then at –20°C. Quality controls were whole blood samples of known Se concentration.18 The two published studies (1977 and 1979 surveys) included whole blood Se analyses only, so plasma Se concentrations in these surveys were estimated as 80% of those for whole blood (Box 2).2
The method of analysis chosen to investigate the effects of age, sex and group (and hence time) for Se data from 1977 to 2002 was a linear mixed model, where “mixed” relates to the inclusion of both fixed and random effects.19 It is assumed that the levels of a random effect arise from a probability distribution. The best linear unbiased predictors (BLUPs) were used to interpret the group effect. The fixed effects were tested with a Wald test, and the random effect with a log-likelihood ratio test. All analyses were performed with S-PLUS 2000.20
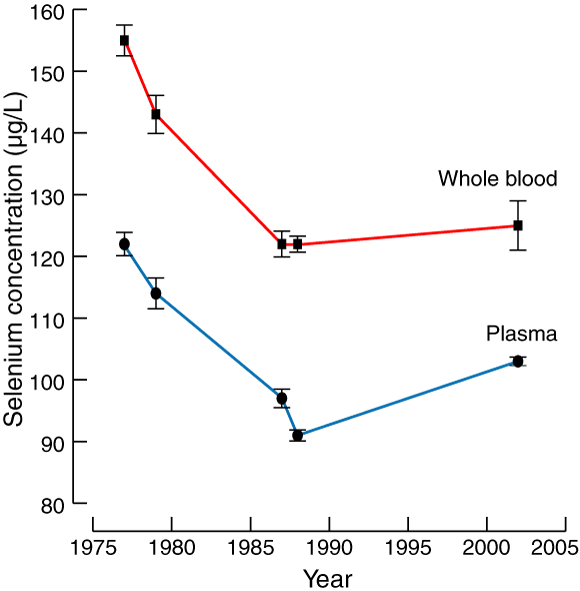
Data from the six surveys examined in this study are summarised in Box 2 and Box 3.
Whole blood Se concentration was affected by survey year (P < 0.05). The mean values in the first two surveys were higher than in the four later surveys. Mean whole blood Se concentration for individuals in the 1977 and 1979 surveys was 153 μg/L, while for the 1987 (2), 1988 and 2002 surveys it was 122 μg/L — a decrease of 20% (see Box 3). There was no interaction effect between age and sex (P = 0.12), and no effect of sex (P = 0.67) or age (P = 0.08).
The major determinant of Se status in humans is the level of available Se in the soil on which their food is grown.1,2 The samples used in this South Australian study comprised relatively healthy adults. Se concentrations in smokers, 21-23 chronically ill people,3 the frail elderly,24 children,25 and pregnant or lactating women26,27 may be 25%–30% lower, and in infants 60% lower,28 than those in adult control subjects.
The mean plasma Se concentration of 103 μg/L found in the sample of Adelaide blood donors in 2002 is higher than that reported from most countries2 (eg, New Zealand [56 μg/L]29 and France [87 μg/L]30), and higher than the mean (93 μg/L) and median (88 μg/L) of Australian published studies.4 However, it is lower than most plasma Se levels reported from the “high Se intake” countries (Venezuela, America, Japan, Norway2 and Canada [135 μg/L]).31 The mean value for the South Australian sample is similar to the plasma Se concentration of 100 μg/L suggested as being necessary for optimal expression of glutathione peroxidase,1 and 39% (111/288) of the sample had Se concentrations below this level.
We found men had a marginally (although statistically significant) higher plasma Se concentration than women; this finding was similar to that of a recent United States study.23 Most studies have found little relationship between sex and blood Se concentration in adults,21 unless women in late-term pregnancy or those who are lactating are included.26,27
The South Australian surveys (which included participants mostly in the 25–65-years age range) found that plasma Se concentration increased significantly with age, while the increase for Se concentration in whole blood was not significant. Most studies have found little difference in plasma and whole blood Se levels in people aged between 20 and 65 years. Our surveys included few individuals aged over 70 years. In people aged over 65 years, plasma Se concentration tends to decrease with age,21 but this change may be related to illness and lower food intake rather than ageing per se.24
Our population samples suggest that a decline in Se concentration of around 20% occurred from the late 1970s to the late 1980s (Box 3). This might have been caused by a decrease in the mean Se concentration in South Australian wheat, given the importance of this source of Se,6 and could have resulted from more intensive cropping, lower soil pH, increased use of gypsum (which contains sulfur) to treat sodic soils, or a combination of these factors.
In conclusion, from 1977 to 2002, in healthy South Australian adults, whole blood and plasma Se concentrations were above those reported for most other countries and in most previous Australian studies, notwithstanding an apparent decline in selenium status from the late 1970s to the late 1980s. However, it is likely that many South Australians do not consume enough Se to maximise selenoenzyme expression. High-risk individuals, including male smokers, men at increased risk of prostate cancer, pregnant or lactating women, infants and the frail elderly, may benefit from Se supplementation, but further studies are needed in these groups. Before recommending fortification or widespread supplementation with Se it would be prudent to await the results of current studies of intervention with Se to examine the effects on cancer, HIV/AIDS and asthma. 1,3,4
1: Some published plasma selenium reference levels
|
15 μg/L | The lowest published level: Burundi, a very low selenium country10 |
|
45 μg/L | Typical level from Bulgaria, a low selenium country11 |
|
89 μg/L | Mean of previous published Australian studies (post-1990 data)4 |
|
100 μg/L | Minimum level for maximisation of glutathione peroxidase activity in plasma1 |
|
113 μg/L | Baseline selenium level below which supplemented selenium protected against cancer in the United States Nutritional Prevention of Cancer Trial6 |
|
120 μg/L | Plausible target selenium level for reduction of cancer risk2 |
|
216 μg/L | Level in a sample from Venezuela, a high-selenium country12 |
2: Characteristics of survey populations and whole blood and plasma selenium concentrations in South Australian residents, 1977–2002
|
Survey |
||||||||||
|
December 1977 |
February 1979 |
October 1987 |
October 1987 |
July 1988 |
June 2002 |
All surveys |
||||
Location |
IMVS* |
Kangaroo Island† |
Adelaide‡ |
Mt Gambier§ |
Adelaide‡ |
Adelaide‡ |
|
||||
No. of participants |
117 |
30 |
96 |
103 |
200 |
288 |
834 |
||||
Men |
70 |
20 |
47 |
59 |
100 |
149 |
445 |
||||
Women |
47 |
10 |
49 |
44 |
100 |
139 |
389 |
||||
Mean age in years (range) |
32 (17–64) |
37 (18–59) |
42 (22–62) |
41 (20–46) |
39 (19–64) |
49 (17–71) |
42 |
||||
Mean selenium concentration (μg/L)†† |
|
|
|
|
|
|
|
||||
Whole blood |
155 (SD, 27) |
143 (SD, 17) |
123 (SD, 20) |
121 (SD, 20) |
122 (SD, 18) |
125¶ (SD, 20) |
128 |
||||
Plasma |
122** (SD, 21) |
114** (SD, 14) |
98 (SD, 15) |
96 (SD, 14) |
91 (SD, 12) |
103 (SD, 11) |
102 |
||||
* Adelaide employees of the Institute of Medical and Veterinary Science.13 † Residents of Kangaroo Island, 70 km south-west of Adelaide.14 ‡ Red Cross blood donors in Adelaide. § Mount Gambier, 400 km south-east of Adelaide. ¶ From 28 people only. ** Estimated values (based on 80% of the measured whole blood Se concentration2). †† Whole blood and plasma selenium concentration means were similar for men and women within each survey. |
|||||||||||
Received 9 May 2003, accepted 13 January 2004
- Graham H Lyons1
- James C R Stangoulis2
- Lyndon T Palmer3
- Robin D Graham4
- Geoffrey J Judson5
- Janine A Jones6
- 1 School of Agriculture and Wine, University of Adelaide, Adelaide, SA.
- 2 Livestock Systems, South Australian Research and Development Institute, Adelaide, SA.
- 3 BiometricsSA, University of Adelaide/South Australia Research and Development Institute, Adelaide, SA.
We thank the Grains Research and Development Corporation for funding; Kathleen Doherty and Sue Heatley (Australian Red Cross Blood Service, Adelaide); Waite Analytical Services; Kevin Mattschoss for his skilled technical assistance (fluorimetric analysis); Patricia Warner (blood centrifugation); and Yusuf Genc and Julia Humphries for assistance with the manuscript.
Graham Lyons is the recipient of a Grains Research and Development Corporation (GRDC) PhD scholarship. The GRDC was not involved in the research, writing or submission of this manuscript.
- 1. Rayman MP. The importance of selenium to human health. Lancet 2000; 356: 233-241.
- 2. Combs GF. Selenium in global food systems. Br J Nutr 2001; 85: 517-547.
- 3. Rayman MP. The argument for increasing selenium intake. Proc Nutr Soc 2002; 61: 203-215.
- 4. Lyons GH, Stangoulis JCR, Graham RD. High-selenium wheat: biofortification for better health. Nutr Res Rev 2003; 16: 45-60.
- 5. Baum MK, Shor-Posner G. Micronutrient status in relationship to mortality in HIV-1 disease. Nutr Rev 1998; 56: S135-S139.
- 6. Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev 2002; 11: 630-639.
- 7. Barrett J, Patterson C, Reilly C, Tinggi U. Selenium in the diet of children with phenylketonuria. In: Southgate DAT, Johnson IT, Fenwick GR, editors. Nutrient availability: chemical and biological aspects. London: Royal Society of Chemistry, 1989: 281-283.
- 8. Frost DV. Why the level of selenium in the food chain appears to be decreasing. In: Combs GF, Levander OA, Spallholz J, editors. Selenium in biology and medicine. Part A. New York: AVI Van Nostrand, 1987: 534-547.
- 9. Dhillon KS, Dhillon SK. Selenium accumulation by sequentially grown wheat and rice as influenced by gypsum application in a seleniferous soil. Plant Soil 2000; 227: 243-248.
- 10. Benemariya H, Robberecht H, Deelstra H. Daily dietary intake of copper, zinc and selenium by different population groups in Burundi, Africa. Sci Total Environ 1993; 136: 49-76.
- 11. Marinov B, Tsachev K, Koleva V. The concentration of selenium in the maternal serum in cases of missed abortion. Akush Ginekologia (Sophia) 1998; 37: 15-16.
- 12. Bratter P, Negretti V, Rosick V, et al. Effect of selenium intake in man at high dietary levels of seleniferous areas of Venezuela. In: Bratter P, Schramel P, editors. Trace element analytical chemistry in medicine and biology. Vol. 3. New York: de Gruyter, 1984: 29-46.
- 13. Judson GJ, Mattschoss KH, Thomas DW. Selenium in whole blood of Adelaide residents. Proc Nutr Soc Aust 1978; 3: 105.
- 14. Judson GJ, Thomas DW, Mattschess KH. Blood selenium levels of Kangaroo Island residents. Med J Aust 1982; 2: 217.
- 15. Tracy MI, Moller G. Continuous flow vapor generation for inductively coupled argon plasma spectrometric analysis. Part 1: Selenium. J Assoc Offic Analytic Chem 1990; 73: 404-410.
- 16. Watkinson JH. Fluorimetric determination of selenium in biological material with 2,3-diaminophthalene. Analytic Chem 1966; 38: 92-97.
- 17. Koh TS, Benson TH. Critical reappraisal of fluorimetric method for determination of selenium in biological materials. J Assoc Offic Analytic Chem 1983; 66: 918-926.
- 18. Koh T-S. Interlaboratory study of blood selenium determinations. J Assoc Offic Analytic Chem 1987; 70: 664-667.
- 19. Searle SR, Casella G, McCulloch CE. Variance components. New York: John Wiley & Sons, 1992: 7-15.
- 20. S-PLUS 2000 [computer program]. Cambridge, MA: Mathsoft Engineering & Education, Inc., 2000.
- 21. Bates CJ, Thane CW, Prentice A, Delves HT. Selenium status and its correlates in a British national diet and nutrition survey: people aged 65 years and over. J Trace Elem Med Biol 2002; 16: 1-8.
- 22. Luty-Frackiewicz A, Jethon Z, Januszewska L. Effect of smoking and alcohol consumption on the serum selenium level of Lower Silesian population. Sci Total Environ 2002; 285: 89-95.
- 23. Kafai MR, Ganji V. Sex, age, geographical location, smoking, and alcohol consumption influence serum selenium concentrations in the USA: third National Health and Nutrition Examination Survey, 1988-1994. J Trace Elem Med Biol 2003; 17: 13-18.
- 24. Campbell A, Bunker V, Thomas A, Clayton B. Selenium and vitamin E status of healthy and institutionalized healthy subjects: analysis of plasma, erythrocytes and platelets. Br J Nutr 1989; 62: 221-227.
- 25. Barany E, Bergdahl IA, Bratteby LE, et al. Relationships between trace element concentrations in human blood and serum. Toxicol Lett 2002; 134: 177-184.
- 26. Reyes H, Baez ME, Gonzalez C, et al. Selenium, zinc and copper plasma levels in intrahepatic cholestasis of pregnancy, in normal pregnancies and in healthy individuals, in Chile. J Hepatol 2000; 32: 542-549.
- 27. Cumming F, Fardy JJ, Woodward DR. Selenium and human lactation in Australia: milk and blood selenium levels in lactating women and selenium intakes of their breast-fed infants. Acta Paediatr 1992; 81: 292-295.
- 28. Daniels LA, Gibson RA, Simmer KN. Indicators of selenium status in Australian infants. J Paediatr Child Health 2000; 36: 370-374.
- 29. Robinson MF, Thomson CD, Jenkins P, et al. Long-term supplementation with selenate and selenomethionine: urinary excretion by New Zealand women. Br J Nutr 1997; 77: 551-563.
- 30. Coudry C, Roussel A, Arnaud J, Favier A. EVA Study Group selenium and antioxidant vitamin and lipoperoxidation levels in pre-aging French population. Biol Trace Elem Res 1997; 57: 183-190.
- 31. Burk KE, Bedford RG, Combs GF, et al. The effect of topical L-selenomethionine on minimal erythema dose of ultraviolet irradiation in humans. Photodermatol Photoimmunol Photomed 1992; 9: 1-6.





Abstract
Objective: To assess trends in selenium status in South Australians from 1977 to 2002.
Design: Six cross-sectional surveys.
Participants: 117 participants in 1977, 30 in 1979, 96 and 103 (separate surveys) in 1987, 200 in 1988, and 288 volunteer blood donors in 2002. A total of 834 healthy Australian adults (mean age, 42 years [range, 17–71 years]; 445 were male).
Main outcome measures: Plasma and whole blood selenium concentrations.
Results: The 2002 survey yielded a mean plasma selenium concentration of 103 μg/L (SE, 0.65), which reached the estimated nutritional adequacy level of 100 μg/L plasma selenium. Mean whole blood selenium declined 20% from the 1977 and 1979 surveys (mean whole blood selenium concentration, 153 μg/L) to the 1987, 1988 and 2002 surveys (mean whole blood selenium concentration, 122 μg/L). Plasma selenium was higher in men (P = 0.01), and increased with age in both men and women (P = 0.008).
Conclusions: In healthy South Australian adults sampled from 1977 to 2002, whole blood and plasma selenium concentrations were above those reported for most other countries and in most previous Australian studies, notwithstanding an apparent decline in selenium status from the late 1970s to the late 1980s.