Barrett’s oesophagus is a premalignant condition, with dysplasia usually preceding the development of adenocarcinoma. Optimal medical management of Barrett’s oesophagus is uncertain. Although there is evidence that anti-reflux surgery results in regression of dysplasia and possibly prevents the development of high-grade dysplasia and adenocarcinoma,1,2 persisting reflux may continue to produce proliferative activity and more dysplasia.3 Evidence of the effectiveness of ablation therapy is scarce, with no evidence that it improves patient outcomes.4 There is speculation that chemoprevention may reduce the incidence of dysplasia and adenocarcinoma, 5-8 warranting trials of chemoprevention with antisecretory therapy combined with cyclo-oxygenase-2 (COX-2) inhibitors.7 However, acid suppression with a proton-pump inhibitor (PPI) alone has been shown to stabilise proliferative cell activity in Barrett’s oesophagus,9-11 and may prevent dysplasia and reduce cancer risk. 8,10,11
Our database of the results of long-term surveillance in patients with Barrett’s oesophagus provided the opportunity to review the natural history of dysplasia both before PPI therapy became available and after its use became widespread. This study aimed to investigate whether management of Barrett’s oesophagus with a PPI reduced the incidence and progression of dysplasia.
The study analysed data from the prospective database of patients at the Brindabella and Mugga Wara Endoscopy Centres, Canberra, ACT. The study was undertaken by three community-based gastroenterologists under guidelines approved by the Australian Capital Territory Health and Community Care Ethics Committee. Data were evaluated in 2001.
The study included all patients diagnosed with Barrett’s oesophagus at the centres between January 1981 and July 2001. Those diagnosed with high-grade dysplasia or adenocarcinoma of the oesophagus at enrolment in the surveillance program were excluded from the study. Follow-up was to 31 July 2001.
Diagnostic criteria for Barrett’s oesophagus were as follows:
Long-segment Barrett’s oesophagus was diagnosed if a segment of columnar-lined lower oesophagus with length ≥ 3 cm was seen at endoscopy, and a biopsy indicated intestinal metaplasia on at least one occasion.
Short-segment Barrett’s oesophagus was diagnosed if a segment of columnar-lined oesophagus with length < 3 cm12 was seen at endoscopy, and biopsy showed intestinal metaplasia on more than one occasion.
Upper-gastrointestinal endoscopies were performed by gastroenterologists, using Olympus or Pentax endoscopes. The positions of endoscopic landmarks were determined in centimetres from the teeth, with documentation of the level of oesophagitis and the squamocolumnar junction, the length of columnar-lined lower oesophageal mucosa and the upper limit of gastric folds.
Oesophagitis was graded initially with the Savary–Miller classification, 13 and later with the Los Angeles classification.14 Severe oesophagitis (grade C or D), nodularity, Barrett’s ulcer (an ulcer within the columnar-lined segment) and stricture were noted as macroscopic markers that may predict dysplasia. Biopsy specimens were taken from areas of ulceration and nodularity, as well as from the whole length of the Barrett’s segment, with care to ensure that all of its quadrants were sampled at least every 2 cm. The origin of each biopsy specimen was not mapped.
Patients with severe oesophagitis or other macroscopic markers had a review endoscopy within 3 to 6 months of diagnosis. Annual surveillance was recommended for all other patients.
A total of 216 patients (62%) began PPI therapy on enrolment in surveillance, while 104 (30%) started this therapy after enrolment, after a median time of 2.2 years (range, 2 weeks to 15 years). The time to starting PPI therapy varied significantly by year of enrolment (Box 1). Thirty people (9%) did not use a PPI during the study.
Types of PPI therapy and dose after stabilisation were:
omeprazole (77% of those who used a PPI) at doses of 20 mg (59%), 40 mg (17%) or 60 mg (1%);
lansoprazole (18%) at doses of 30 mg (14%), 60 mg (3%) or 90 mg (1%); and
pantoprazole (5%) at a dose of 40 mg.
Seventy-eight patients (22%) used either aspirin (43) or an NSAID (35). Seventy-one of these (91%) also used a PPI at some time. Among the 30 patients who did not use a PPI during the study, 23 also had no record of taking aspirin or NSAIDs.
The relationship between use of PPI therapy and time to onset of low-grade dysplasia was examined in the 299 patients who were free of this condition at enrolment. Use of PPI therapy was significantly related to the time to onset of low-grade dysplasia (log rank test = 43.6, df = 1, P < 0.001; Box 2). The 42 patients who did not use a PPI before being diagnosed with low-grade dysplasia had a mean time to this diagnosis of 8.8 years (95% CI, 6.2–11.5); 50% developed the condition within 3.2 years of enrolment. The 257 patients who used a PPI before diagnosis of low-grade dysplasia or censoring had a mean time to this diagnosis of 14.1 years (95% CI, 13.1–15.0); only 19% developed the condition during surveillance.
To examine further the effect of delayed PPI use on the onset of low-grade dysplasia, and to adjust for confounders, we included the variables age, sex, presence of macroscopic markers, and use of aspirin or NSAIDs in a Cox regression analysis stratified by time of enrolment (Box 3). Patients who delayed PPI therapy for 2 years or more had 5.6 times (95% CI, 2.0–15.7) the risk of developing low-grade dysplasia at a given time as those who used a PPI within the first year of entering the surveillance program. Age and the presence of macroscopic markers on enrolment were also significantly related to onset of low-grade dysplasia. For instance, patients with a macroscopic marker on enrolment had 3.4 times (95% CI, 1.9–6.1) the risk of developing low-grade dysplasia at a given time compared with those with no markers present.
Although only 11 patients developed high-grade dysplasia or adenocarcinoma, we found a significant relationship between the delay before PPI use and time to onset of these conditions (Box 3). After considering confounders and stratifying for period of enrolment in surveillance in a Cox regression analysis, we found that patients who delayed the use of a PPI for 2 years or more after diagnosis had 20.9 times (95% CI, 2.8–158) the risk of developing high-grade dysplasia or adenocarcinoma at a given time compared with those who used a PPI within the first 2 years of enrolment.
Also, sex and the presence of macroscopic markers at enrolment were significantly related to onset of high-grade dysplasia or adenocarcinoma (Box 3). For example, patients with a marker present had 33.4 times (95% CI, 3.8–289) the risk of developing high-grade dysplasia or adenocarcinoma at a given time compared with those with no marker present at enrolment.
There was also a tendency for women to have a smaller risk of developing high-grade dysplasia or adenocarcinoma at any given time than men (hazard ratio, 0.21; 95% CI, 0.04–1.10).
Similar results were obtained when the analysis was restricted to patients with low-grade dysplasia. Those who delayed PPI therapy for 2.5 years or longer had 5.1 times (95% CI, 0.8–33.7) the risk of developing high-grade dysplasia or adenocarcinoma (8 patients) at a given time compared with those who started PPI therapy within 2.5 years of enrolment. However, because of the small numbers involved, these results should be treated with caution.
We found that PPI therapy appeared beneficial in preventing the development of low-grade dysplasia in Barrett’s oesophagus. PPI therapy also significantly reduced the development of high-grade dysplasia or adenocarcinoma. Use of a PPI varied by the year of diagnosis with Barrett’s oesophagus, but, after stratification by year and other variables, starting PPI therapy within 2 years of diagnosis was beneficial in preventing the development and progression of dysplasia. We found that patients of similar age, enrolling with similar profiles of macroscopic markers, had a significantly increased risk of low-grade dysplasia if the use of PPI was delayed by 2 years or more after diagnosis.
This study confirms our observation that fewer patients with Barrett’s oesophagus developed dysplasia after the introduction of PPI therapy in Australia in 1989. We postulated that the incidence of dysplasia was influenced by powerful acid suppression that reduced oesophageal acid exposure.
Mucosal acid exposure has been shown to promote epithelial proliferation.3,11,15 A study of the effect of acid exposure in vitro and in vivo confirmed that even brief acid exposure significantly increases cell proliferation and possibly decreases apoptosis in Barrett’s oesophagus.15 Increased epithelial proliferation in patients with Barrett’s oesophagus has been associated with a stepwise progression of dysplasia to adenocarcinoma. 3,8,10 Other studies have suggested that COX-2 is overexpressed in the early transformation of oesophageal epithelium in Barrett’s oesophagus and in the transition from low- to high-grade dysplasia and adenocarcinoma.6,16,17
While acid suppression using PPI therapy has been shown to stabilise proliferative cell activity in Barrett’s oesophagus, 9-11 and, at maximum doses,18,19 to decrease the length of Barrett’s oesophagus, there is no evidence that it completely reverses the condition.5,7,10,11 However, it has been argued that the central concern is not reversing Barrett’s oesophagus, but rather preventing the development of related malignancy, which might be influenced by powerful antisecretory therapy and COX-2 inhibition.5 Most studies in this field stress the need for more research and evaluation of different strategies, while acknowledging the difficulty of obtaining large numbers of patients for long-term studies.5,10,17,20
Our study was large, involving 350 patients, some of whom were followed up for more than 20 years. While this study does not have the soundness of a randomised controlled trial, it does offer the opportunity to review the long-term outcomes of PPI therapy. Although only 11 patients developed high-grade dysplasia or adenocarcinoma, the presence of low-grade dysplasia was also a meaningful primary outcome measure, as this is predictive of cancer12,21-23 and influences clinical surveillance decisions. Active inflammation may influence diagnosis of low-grade dysplasia because of cell atypia, 8,12 and therefore dysplasia was reported only in the absence of inflammation. We have argued elsewhere that the presence of macroscopic markers at diagnosis is also predictive of high-grade dysplasia and adenocarcinoma,24 and this was also included in the analysis.
The date of starting PPI therapy was recorded for each patient in this study. The degree of acid suppression was not measured, but PPI therapy was offered to all patients with Barrett’s oesophagus and was continued long term, rather than being discontinued or the dose reduced when symptoms were controlled.2 Current studies suggest that the use of aspirin and NSAIDs may also reduce the risk of oesophageal cancer.25 However, we were not able to draw conclusions about this, as use of these drugs was poorly documented in the first decade of the study and did not contribute significantly to outcomes in the model.
Is it necessary to introduce a COX-2 inhibitor for chemoprotection? Exposing metaplastic epithelium to acid results in the expression of cyclo-oxygenase, an anti-apoptotic protein.15 It is theoretically attractive to use a COX-2 inhibitor to counter this, and COX inhibition has been shown to reduce the incidence of adenocarcinoma in a rat model.6 However, if PPI therapy, which has proven safe in the long term,26 reduces acid exposure and prevents the development of dysplasia, further agents may not be needed. The results of our study suggest that it may be beneficial to encourage all patients with Barrett’s oesophagus to continue using PPI therapy in the long term to prevent dysplasia, even if they have no oesophagitis or symptoms.
1: Percentage of patients who used proton-pump inhibitors, by year of enrolment in surveillance (n = 350)
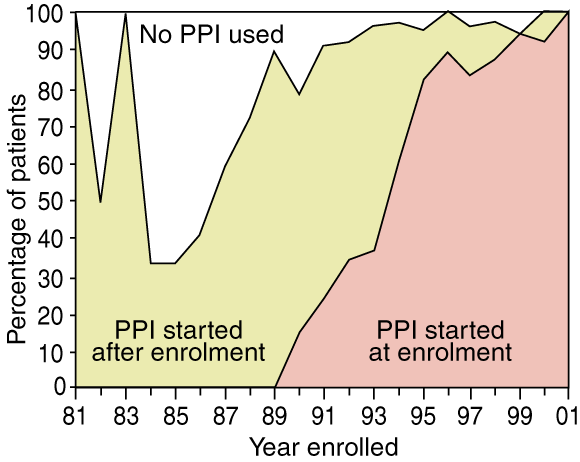
PPI = proton-pump inhibitor. Proportion taking PPIs varied greatly between 1981 and 1985 because of the small number of patients entering surveillance.
2: Kaplan–Meier curves of the cumulative proportion of patients who were free of low-grade dysplasia (n = 299*)
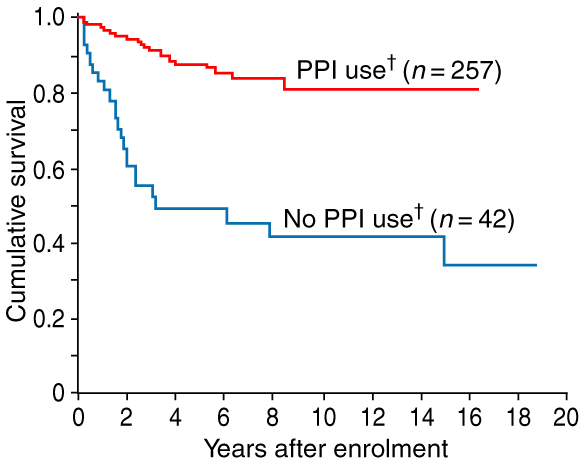
PPI = proton-pump inhibitor. * 51 patients were excluded from this analysis, as they had low-grade dysplasia on enrolment in surveillance. † Use of a PPI was defined as a recorded date of use before diagnosis of low-grade dysplasia or the censor date of 31 July 2001.
3: Significant predictors of time to onset of dysplasia or cancer among patients with Barrett’s oesophagus, by Cox regression analysis*
Predictor |
Regression coefficient (SE) |
Hazard ratio (95% CI) |
P |
||||||||
Low-grade dysplasia (n = 299)† |
|
|
|
||||||||
Age at enrolment (10-year groups) |
0.03 (0.01) |
1.03 (1.00–1.05) |
0.03 |
||||||||
Macroscopic marker on enrolment‡ |
1.23 (0.30) |
3.40 (1.89–6.12) |
< 0.001 |
||||||||
Time to PPI use from enrolment§ |
|
|
0.004 |
||||||||
< 1 year |
1.0 |
|
|
||||||||
1 to < 2 years |
0.26 (0.75) |
1.30 (0.30–5.68) |
0.73 |
||||||||
≥ 2 years |
1.73 (0.52) |
5.64 (2.02–15.7) |
0.001 |
||||||||
High-grade dysplasia or cancer (n = 328)¶ |
|
|
|
||||||||
Female sex |
− 1.57 (0.85) |
0.21 (0.04–1.10) |
0.06 |
||||||||
Macroscopic marker on enrolment ‡ |
3.51 (1.10) |
33.4 (3.85–289) |
0.001 |
||||||||
Time to PPI use from enrolment ≥ 2 y§ |
3.04 (1.00) |
20.9 (2.78–158) |
0.003 |
||||||||
* Cox regression was stratified by period of enrolment (1980–1990, 1991–1994, 1995–2001) to account for period effects. † 51 patients were excluded from the analysis, as they had low-grade dysplasia on enrolment in surveillance. ‡ Macroscopic markers included severe oesophagitis, stricture, Barrett’s ulcer and nodularity. § Time-dependent covariate. ¶ Twenty-two patients were excluded from the analysis because of censoring before the earliest event in the stratum. |
|||||||||||
Received 17 October 2003, accepted 23 October 2003
- Lybus C Hillman1
- Louise Chiragakis2
- Graham L Kaye3
- Anthony C Clarke4
- Bruce Shadbolt5
- 1 Mugga Wara and Brindabella Endoscopy Centres, Canberra, ACT.
- 2 Department of Epidemiology, The Canberra Hospital, Canberra, ACT.
We thank the gastroenterologists and nursing staff from Brindabella and Mugga Wara Endoscopy Centres for providing the database for this study, gastrotrACT for funding the research, and Dr Sanjiv Jain for his critical role in defining the pathological changes. We are also grateful to the anonymous reviewers of the article, whose comments allowed us to improve it significantly.
None identified.
- 1. Hofstetter WL, Peters JH, DeMeester TR, et al. Long-term outcome of antireflux surgery in patients with Barrett’s esophagus. Ann Surg 2001; 234: 532-539.
- 2. Koop H. Gastroesophageal reflux disease and Barrett’s esophagus. Endoscopy 2002; 34: 97-103.
- 3. Chen L, Hu C, Gaboury L, et al. Proliferative activity in Barrett’s esophagus before and after antireflux surgery. Ann Surg 2001; 234: 172-180.
- 4. Gross CP, Cruz-Correa M, Canto MI, et al. The adoption of ablation therapy for Barrett’s esophagus: a cohort study of gastroenterologists. Am J Gastroenterol 2002; 97: 281-286.
- 5. Fennerty MB, Triadafilopoulos G. Barrett’s related adenocarcinoma: is chemoprevention a potential option? Am J Gastroenterol 2001; 96: 2302-2305.
- 6. Buttar NS, Wang KK, Leontovich O, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett’s esophagus. Gastroenterology 2002; 122: 1101-1112.
- 7. Fennerty MB. Barrett’s-related esophageal cancer: has the final hurdle been cleared, now paving the way for human chemoprevention trials? Gastroenterology 2002; 122: 1172-1175.
- 8. Falk GW. Barrett’s esophagus. Gastroenterology 2002; 122: 1569-1591.
- 9. Umansky M, Yasui W, Hallak A, et al. Proton pump inhibitors reduce cell cycle abnormalities in Barrett’s esophagus. Oncogene 2001; 20: 7987-7991.
- 10. Peters FTM, Ganesh S, Kuipers EJ, et al. Effect of elimination of acid reflux on epithelial cell proliferative activity of Barrett esophagus. Scand J Gastroenterol 2000; 12: 1239-1244.
- 11. Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology 1999; 117: 327-335.
- 12. Sampliner RE and the Practice Parameters Committee of the American College of Gastroenterology. Practice guidelines on the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol 1998; 93: 1028-1032.
- 13. Savary M, Miller G. L’oesophage. Manuel et atlas d’endoscopie. Solieure: Gassman, 1977.
- 14. Armstrong D, Bennett JR, Blum AL, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology 1996; 111: 85-92.
- 15. Souza RF, Shewmake K, Terada LS, Spechler SJ. Acid exposure activates the mitogen-activated protein kinase pathways in Barrett’s esophagus. Gastroenterology 2002; 122: 299-307.
- 16. Shirvani VN, Ouatu-Lascar R, Kaur BS, et al. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology 2000; 118: 487-496.
- 17. Morris CD, Armstrong GR, Bigley G, et al. Cyclooxygenase-2 expression in the Barrett’s metaplasia-dysplasia-adenocarcinoma sequence. Am J Gastroenterol 2001; 96: 990-996.
- 18. Peters FTM, Ganesh S, Kuipers EJ, et al. Endoscopic regression of Barrett’s esophagus during omeprazole treatment; a randomised double blind study. Gut 1999; 45: 489-494.
- 19. Srinivasan R, Katz PO, Ramakrishnan A, et al. Maximal acid reflux control for Barrett’s esophagus: feasible and effective. Aliment Pharmacol Ther 2001; 15: 519-524.
- 20. Weinstein MW. The prevention and treatment of dysplasia in gastroesophageal reflux disease: The results and the challenges ahead. J Gastroenterol Hepatol 2002; 17: S113-S124.
- 21. Hameeteman W, Tytgat GNJ, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology 1989; 96: 1249-1256.
- 22. van Sandick JW, van Lanschot JJB, Kuiken BW, et al. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut 1998; 43: 216-222.
- 23. Rusch VW, Levine DS, Haggitt R, Reid BJ. The management of high grade dysplasia and early cancer in Barrett’s esophagus. Cancer 1994; 74: 1225-1229.
- 24. Hillman LC, Chiragakis L, Clarke AC, et al. Barrett’s esophagus: macroscopic markers and the prediction of dysplasia and adenocarcinoma. J Gastrenterol Hepatol 2003; 18: 526-533.
- 25. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology 2003; 124: 47-56.
- 26. Klinkenberg-Knol EC, Nelis F, Dent J, et al. Long-term omeprazole treatment in resistant gastroesophageal reflux disease: efficacy, safety, and the influence on gastric mucosa. Gastroenterology 2000; 118: 661-669.





Abstract
Objective: To examine whether proton-pump inhibitor (PPI) therapy influences the incidence and progression of dysplasia in patients with Barrett’s oesophagus.
Design and setting: Review of prospective data on patients undergoing surveillance with regular endoscopy and biopsy at a private endoscopy centre in Canberra, ACT, between 1981 and 2001.
Patients: 350 patients diagnosed with Barrett’s oesophagus.
Interventions: PPI therapy was progressively introduced into clinical practice from late 1989. Once begun, PPI therapy was ongoing, with no attempt to reduce the dose.
Main outcome measures: Relationship between development of dysplasia or adenocarcinoma and delay between diagnosis with Barrett’s oesophagus and starting PPI therapy was determined by Cox regression analyses, stratified by year of enrolment. Age, sex, presence of macroscopic markers (severe oesophagitis, nodularity, Barrett’s ulcer, stricture) and use of aspirin or non-steroidal anti-inflammatory drugs were considered as confounding factors in the regression analyses.
Results: The 350 patients had 1422 surveillance endoscopies, with a median follow-up of 4.7 years. Patients who delayed using a PPI for 2 years or more after diagnosis with Barrett’s oesophagus had 5.6 times (95% CI, 2.0–15.7) the risk of developing low-grade dysplasia at any given time as those who used a PPI in the first year. Similar results were found for the risk of developing high-grade dysplasia or adenocarcinoma (hazard ratio, 20.9; 95% CI, 2.8–158).
Conclusions: Use of ongoing PPI therapy appeared beneficial in the prevention of dysplasia and adenocarcinoma in patients with Barrett’s oesophagus. We suggest that all patients with this condition, even those with no oesophagitis or symptoms, should be encouraged to continue long term PPI therapy.