Creutzfeldt–Jakob disease (CJD) is a fatal, transmissible, neurodegenerative disorder belonging to the group known as the transmissible spongiform encephalopathies (TSEs). CJD can occur without explanation (sporadic), secondary to mutations in the prion protein gene (PRNP), or as a complication of medical treatment using contaminated therapeutic agents or equipment (iatrogenic). Although corneal grafts and neurosurgical equipment have been associated with disease transmission, the most common causes of iatrogenic CJD have been treatments involving human-derived cadaveric pituitary hormones or dura mater.1
The first identified case of CJD in a dura mater recipient was reported in the United States in early 1987.2 In response, the US Food and Drug Authority issued a safety alert in April 1987, seeking immediate discontinuation of use of the identified dura mater batch (Lyodura batch #2105).3 A second patient with CJD linked to Lyodura was detected in New Zealand in 1988,4 but the specific batch could not be identified. This has remained a frequent difficulty when tracing contamination sources.
As of January 2003, over 120 CJD cases related to dura mater use had been detected globally, with 97 in Japan.5 These cases were predominantly associated with Lyodura, a commercial product produced since 1969 by B Braun Melsungen AG (based in Germany). Only a few reports suggest the possibility of CJD after use of dura mater from other commercial or non-commercial sources.6-8
Lyodura consists of lyophilised, irradiated human dura mater sourced post mortem. Additional processing with immersion in a solution of sodium hydroxide (1 M) was instituted in 1987, with a noticeable reduction in Lyodura-related cases thereafter.9 Lyodura has been used in a number of countries, including Australia, Japan, Canada, the United States and the United Kingdom, mainly in neurosurgery, but also in orthopaedic, otological, dental, urological, gynaecological and cardiac procedures (Box 1) (Dr L Schonberger, Assistant Director, National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga, USA, personal communication).
Most cases of CJD associated with dura mater have occurred in Japan. In 1996, in response to the growing incidence, a survey was undertaken of Lyodura use in almost 3000 Japanese healthcare institutions. This estimated that up to 100 000 people received Lyodura grafts between 1983 and 1987,9,10 and up to 220 000 between 1979 and 1991 (out of a total of 260 000 who received dura mater grafts). Use of these grafts greatly declined after 1991 but may have continued until 1997.11,12 Assuming that all cases of CJD associated with dura mater were a consequence of Lyodura use, the overall risk of Lyodura-associated CJD in Japan is approximately 0.04%.5
Lyodura was approved by the Australian Therapeutic Goods Administration for importation and use in Australia in 1972. The product licence was withdrawn in early May 1987, shortly after recognition of the first case of CJD linked to Lyodura use.
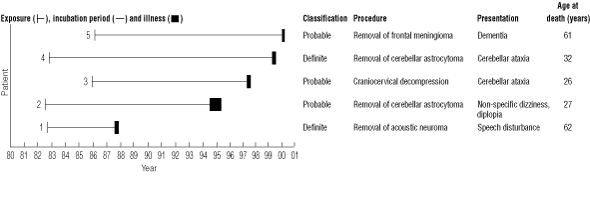
To date, five cases of CJD have been epidemiologically linked to neurosurgical use of Lyodura in Australia. Their clinical features have already been described13-15 and are summarised in Box 2. Patient 1 presented in 1987, about 5 years after implantation of Lyodura. The longest incubation period was in Patient 4, who presented in 1999 after an incubation period of almost 17 years.14 The most recent (fifth) patient died in 2000. Patients 1, 2 and 4 were exposed in 1982, while Patients 3 and 5 were exposed in 1985 and 1986, respectively.
A number of studies have attempted to determine the number of people exposed to Lyodura in Australia. We collated the available information and undertook further enquiries, as a basis for estimating the risk of Lyodura-associated CJD in this country (Box 3).
Quantifying past use of Lyodura in Australia relies on data that cannot be fully confirmed. Initial estimates by the Commonwealth Department of Health and Ageing in discussion with the Therapeutic Goods Administration placed an upper limit of between 5000 and 10 000 individuals potentially exposed to Lyodura. However, from the results reported here, the true number is likely to be much smaller — probably fewer than 2500. If so, the risk of Lyodura-associated CJD is higher in Australia (0.20%–0.23%) than in other countries that have undertaken similar investigations, such as Japan.
Although the risk of Lyodura-associated CJD in Japan has appeared to fluctuate over time, two relatively stable features are a peak in contaminated grafts between 1983 and 1987, and the paucity of Lyodura-associated cases after 1987, when the manufacturer instituted effective decontamination of the tissue with a 1 M solution of sodium hydroxide.1,5,9 Ninety-seven cases associated with dura mater had been recognised to 2003,5 with predictions that further cases are likely until 2020, and that final numbers may reach 160.12 Acknowledging a total of 220 000 people exposed to Lyodura, and assuming all CJD associated with dura mater was associated with Lyodura (which is reasonable based on evidence published to date),11 then the cumulative overall risk of CJD from Lyodura in Japan is currently around 0.04% (95% CI, 0.03%–0.05%) (Box 4). Estimated risk in Australia is much higher — 0.20% to 0.23% (95% CIs, 0.06%–0.47% and 0.07%–0.53%, respectively). With the same assumptions, the risk of CJD in the higher-risk period in Japan (1983–1987) is about 0.08% (95% CI, 0.06%–0.10%), while that in Australia (1982–1986) is 0.43% (95% CI, 0.14%–0.99%). Notwithstanding the need for certain assumptions to facilitate these comparisons, the risk in Australia appears about five times greater than the analogous point estimates of risk in Japan. The reality of the differences is further supported by the lack of overlap between the confidence intervals of the calculated estimates.
Nevertheless, the risk of CJD associated with Lyodura in Australia is well below the risk associated with exposure to human-derived pituitary growth hormone (hGH) in France (Box 4). As of 1999, 55 cases of CJD had been linked to French-derived hGH, from a cohort of 1361 patients, representing an attack rate of about 1 in 25 recipients (4.0%).17 A more recent report suggested the risk could be much higher, at 81 per 1361 (about 6.0%).18
The reason for the higher estimated risk of Lyodura-associated CJD in Australia compared with Japan is not known. Perhaps most likely is chance receipt of a relatively high percentage of contaminated batches of Lyodura and use of multiple pieces of Lyodura per patient in Australia, although the latter is contrary to anecdotal recollections of Australian neurosurgeons. Alternatively, the difference may reflect better case ascertainment in Australia, which, in contrast to Japan, had an established comprehensive, prospective, national surveillance program for CJD, with international comparisons of incidence rates for sporadic CJD attesting to the adequacy of case ascertainment.14
Another potential explanation is genetic difference between the populations of the two countries. For example, homozygosity for methionine at codon 129 of the PRNP gene appears to predispose to iatrogenic CJD.1 However, contrary to expectations, this homozygosity appears more common in the Japanese population (about 92%) than in occidental populations (about 37%).19
Finally, the differences in risk may reflect greater use of Lyodura in Japanese patients with malignancies, whose survival was shorter than the lengthy incubation periods typical of TSEs, or in non-neurosurgical applications, with attendant lower transmission efficiency. Japanese studies suggest that Lyodura-related transmissions have been essentially restricted to neurosurgical procedures,10,11 and our comparative risk analysis was predicated on the assumption that most Lyodura was used in these procedures in both countries. Although some Lyodura was most likely used in non-neurological procedures, the proportion is impossible to quantify in different countries, leaving uncertainty about our risk comparisons.
In addition to neurosurgery and the non-neurological applications identified by Australian surveys, additional uses of Lyodura have been reported in the US (Box 1). Cases of CJD have been linked epidemiologically to Lyodura used in the embolisation of the external carotid artery for treating a nasopharyngeal angiofibroma,20 as well as dura mater used to embolise intercostal arteries before thoracic surgery.7 This reinforces the likelihood that non-neurosurgical use of Lyodura can result in transmission.10 Therefore, risk of Lyodura-associated CJD linked to non-neurosurgical uses appears a genuine possibility, which may be very difficult to identify epidemiologically, given the often deficient state of medical records.
The inability to clearly identify surgical uses of Lyodura, especially non-neurosurgical uses, also raises the possibility of secondary iatrogenic transmissions from Lyodura-treated patients during an extended preclinical or incubation phase. Animal models clearly support the possibility of lengthy presymptomatic periods (which may even exceed the lifespan of hosts) during which transmission is possible.21-24 Generally, infection control and other guidelines focus on the need to limit transmission risk through identifying patients who received Lyodura during neurosurgery, and do not include guidance on risk associated with other surgical applications. This approach may need to be reconsidered based on studies such as ours.
1: Non-neurosurgical uses of Lyodura in the United States*
Development of ligaments to stabilise shoulder joints
Replacement of the tracheal wall
Covering pleura defects
Securing bronchial stumps
Repair of pericardium
Repair of diaphragm defects (traumatic or congenital)
Arthroplasties of the elbow
Reinforcement of fascia in abdominal hernias
Reinforcement of tendons or ligaments
Plastic enlargement of the urinary bladder
Other miscellaneous surgical uses
* Based on information from the US National Center for Infectious Diseases, Centers for Disease Control and Prevention, Atlanta, Ga.
3: Studies of Lyodura use in Australia
Survey of hospitals in Victoria In 1987, after notification of the first patient with Lyodura-associated Creutzfeldt–Jakob disease (CJD), the Victorian Department of Community Services and Health surveyed use of Lyodura in all private and public hospitals in Victoria. Five hospitals responded, out of an uncertain total number. They reported use of 40 Lyodura grafts in 1985–1986 and 36 in 1986–1987, all in neurosurgical procedures (unpublished data). Survey of neurosurgeons In 1995, the Neurosurgical Society of Australasia surveyed 100 neurosurgeons (comprising all practising neurosurgeons and retired surgeons still on their register) about use of Lyodura or other dura mater during their practice lifetimes.13 This survey was also used to assess the feasibility of tracing recipients. Sixty-five neurosurgeons responded (response rate, 65%); 35 of these (54%) had used dura mater grafts in cranial or spinal procedures at some time, with 34 having used Lyodura. Eleven of the 34 (32%) believed that they could identify over 90% of recipients from their records, nine (27%) that they could identify 50%–90%, and 14 that they could identify fewer than 50%. The survey could not determine the precise amount of Lyodura or specific batches used, nor the number of procedures involving Lyodura. However, all three cases of Lyodura-related CJD recognised before the survey were in patients of respondents who reported using Lyodura. The remaining two confirmed cases presented after this survey. Survey of non-neurosurgical use In 2001, the Department of Health and Aged Care (DHAC) undertook a survey through the Royal Australasian College of Surgeons (RACS) to assess non-neurosurgical use of Lyodura. Over 5000 practising and retired surgeons across 15 surgical specialties were asked about use of dura mater grafts (and specifically Lyodura) over their practice lifetimes. Responses were received from 172 surgeons (response rate, 3.4%). This very poor response rate precluded meaningful analysis. However, the survey confirmed that Lyodura had been used in otorhinolaryngological procedures, such as tympanoplasty, myringoplasty and mastoidectomy, with respondents reporting use of about 100 grafts in such applications. Given the poor response rate, time elapsed since the product was withdrawn, recall bias and retirement of surgeons during this time, this may be a significant underestimate of use in non-neurosurgical applications. This possibility is supported by the US Centers for Disease Control and Prevention report that up to 20% of Lyodura use in the US was in non-neurosurgical applications (Dr L Schonberger, personal communication). Quantification study In 2002, the Department of Health and Ageing, in conjunction with the Australian National Creutzfeldt–Jakob Disease Registry, undertook a study to quantify Lyodura use in Australia and to determine the types of procedures in which it was used, as a basis for estimating the risk of Lyodura-associated CJD. Methods: The Registry has ethical approval for its surveillance methods and activities from the University of Melbourne Human Research Ethics Committee. As well as collating results of previous surveys, we asked the manufacturer of Lyodura, B Braun Melsungen AG, about supplies of Lyodura to Australia, including batch numbers and the total quantity of Lyodura imported into and distributed within the country. We also contacted other potential sources of information on Lyodura use, including the Australian Therapeutic Goods Administration and the Health Insurance Commission. Results: Braun Melsungen indicated that:
According to the 1985 Braun catalogue for Australia, Lyodura was available in a range of sizes, with some packs containing up to 6 pieces. The manufacturer indicated that packs sold in Australia contained either one large sheet or two smaller sheets. Its information suggests that generally the larger sheets were used for neurosurgery, and smaller sheets for other surgical applications. The single-sheet packs outsold the double-sheet packs by about three to one. Braun did not supply documentary verification of this information. Neither the Australian Therapeutic Goods Administration nor the Health Insurance Commission could furnish further information on Lyodura use. Based on the manufacturer’s information, and assuming that all distributed product was used, then a maximum of 750 sheets may have been used in Australia between 1978 and 1982. Before 1978, assuming the same distribution of pack sizes, then 180–450 sheets may have been distributed. These data suggest a total use in Australia of 2208 to 2478 Lyodura grafts. Assuming maximum and equal annual use, then about 1172 Lyodura grafts were used in the higher-risk period 1982–1986. Risk estimation: Risk estimates for Lyodura-associated CJD were based on data from the manufacturer alone, as survey results were very incomplete, and Lyodura use reported in the surveys was likely to be encompassed by the manufacturer’s information. Risk estimates were calculated from the total number of individuals exposed and the number of CJD cases detected over defined periods, with 95% confidence intervals calculated using the Poisson distribution. Based on the five Lyodura-associated CJD cases detected to 2003, the attack rate ranged from 1 in 496 patients who received Lyodura (0.20%) to 1 in 442 (0.23%) (Box 4), depending on whether a higher or lower estimate of pre-1978 use was used. In the higher-risk period (1982–1986), the attack rate may have been as high as 1 in 234 (0.43%). These calculations were based on exposure through any surgical application of Lyodura, assuming that only one sheet was used per patient and that all distributed product was used. The latter fact cannot be verified, and previous reports suggest that not all distributed Lyodura was necessarily used.16 These attack rates are therefore likely to be underestimates. The attack rates after neurosurgery in Australia may be even higher, depending on the proportion of Lyodura that was used in this type of surgery. Should the proportion be close to 60% of the total product used (as suggested by the manufacturer) or 80% (as found in the US), the overall neurosurgical attack rate may be as high as 1 in 397 (ie, 80% of 496) to 1 in 265 (ie, 60% of 442), or 0.25% to 0.38%. The CJD Registry also recently reviewed all records to determine whether any patients on the CJD register might have been exposed to Lyodura through non-neurosurgical applications. No potential cases were identified. |
4: Risks of Creutzfeldt–Jakob disease (CJD) related to iatrogenic exposures in different countries
Country |
Period |
Deaths from CJD* |
Recipients† |
Risk (95% CI) |
|||||||
Lyodura |
|
|
|
||||||||
Japan |
Overall (1979–2003) |
97 |
220 000 |
0.04% (0.03%–0.05%) |
|||||||
|
Higher-risk (1983–1987) |
81 |
100 000 |
0.08% (0.06%–0.10%) |
|||||||
Australia |
Overall (pre-1978–2003) |
5 |
2 208‡ |
0.23% (0.07%–0.53%)‡ |
|||||||
|
|
|
2 478‡ |
0.20% (0.06%–0.47%)‡ |
|||||||
|
Higher-risk (1982–1986) |
5 |
1 172 |
0.43% (0.14%–0.99%) |
|||||||
Human growth hormone |
|
|
|||||||||
France |
To 1999 |
55 |
1 361 |
4.04% (3.04%–5.26%) |
|||||||
* Based on cases of CJD with a history of recognised iatrogenic exposure during the defined period. † Based on estimated total national use of Lyodura or human cadaveric pituitary growth hormone during the defined period. ‡ A range exists for the number of Australian Lyodura recipients because of uncertainty about use before 1978. |
|||||||||||
- Fiona J Brooke1
- Alison Boyd2
- Genevieve M Klug3
- Colin L Masters4
- Steven J Collins5
- 1 Communicable Diseases Branch, Department of Health and Ageing, Canberra, ACT.
- 2 Australian National Creutzfeldt–Jakob Disease Registry, Department of Pathology, University of Melbourne, Melbourne, VIC.
The Australian National Creutzfeldt–Jakob Disease Registry is funded by the Australian Department of Health and Ageing. The authors thank Associate Professor Peter Reilly (University of Adelaide) for many details of the Neurosurgical Society of Australasia survey of neurosurgeons, Dr L Schonberger (Centers for Disease Control and Prevention, USA) for estimated percentages for non-neurosurgical use of Lyodura, and Professor Emeritus Donald Simpson, Department of Neurosurgery, Royal Adelaide Hospital, SA, for assistance in reporting and evaluating patients with Lyodura-related CJD.
None identified.
- 1. Brown P, Preece M, Brandel JP, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology 2000; 55: 1075-1081.
- 2. Update: Creutzfeldt-Jakob disease in a patient receiving a cadaveric dura mater graft. MMWR Morb Mortal Wkly Rep 1987; 36: 324-325.
- 3. Food and Drug Administration. FDA safety alert: possibly contaminated dura mater transplant material. Rockville, Md: US Department of Health and Human Services, Public Health Service, Apr 28 1987.
- 4. Update: Creutzfeldt-Jakob disease in a second patient who received a cadaveric dura mater graft. MMWR Morb Mortal Wkly Rep 1989; 38: 37-38.
- 5. Nakamura Y, Watanabe M, Nagoshi K, et al. Update: Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts — Japan, 1979–2003. MMWR Morb Mortal Wkly Rep 2003; 52: 1179-1181.
- 6. Hannah EL, Belay ED, Gambetti P, et al. Creutzfeldt-Jakob disease after receipt of a previously unimplicated brand of dura mater graft. Neurology 2001; 56: 1080-1083.
- 7. Defebvre L, Destee A, Caron J, et al. Creutzfeldt-Jakob disease after an embolization of intercostal arteries with cadaveric dura mater suggesting a systemic transmission of the prion agent. Neurology 1997; 48: 1470-1471.
- 8. Dobbins JG, Belay ED, Malecki J, et al. Creutzfeldt-Jakob disease in a recipient of a dura mater graft processed in the US: cause or coincidence? Neuroepidemiology 2000; 19: 62-66.
- 9. Nakamura Y, Aso E, Yanagawa H. Relative risk of Creutzfeldt-Jakob disease with cadaveric dura transplantation in Japan. Neurology 1999; 53: 218-220.
- 10. Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts — Japan, January 1979–May 1996. MMWR Morb Mortal Wkly Rep 1997; 46: 1066-1069.
- 11. Hoshi K, Yoshino H, Urata J, et al. Creutzfeldt-Jakob disease associated with cadaveric dura mater grafts in Japan. Neurology 2000; 55: 718-721.
- 12. Hamada C, Sadaike T, Fukushima M. Projection of Creutzfeldt-Jakob Disease frequency based on cadaveric dura transplantation in Japan. Neuroepidemiology 2003; 22: 57-64.
- 13. Boyd A, Fletcher A, Lee JS, et al. Transmissible spongiform encephalopathies in Australia. Commun Dis Intell 2001; 25: 248-252.
- 14. Collins S, Boyd A, Lee JS, et al. Creutzfeldt-Jakob disease in Australia 1970-1999. Neurology 2002; 59: 1365-1371.
- 15. Simpson D, Masters CL, Ohlrich G, et al. Iatrogenic Creutzfeldt-Jakob disease and its neurosurgical implications. J Clin Neurosci 1996; 3: 118-123.
- 16. Newcombe RL. Neurosurgery and iatrogenic transmission of Creutzfeldt-Jakob disease. Med J Aust 1996; 164: 603-604.
- 17. Huillard d’Aignaux J, Costagliola D, et al. Incubation period of Creutzfeldt-Jakob disease in human growth hormone recipients in France. Neurology 1999; 53: 1197-1201.
- 18. Brandel J-P, Preece M, Brown P, et al. Distribution of codon 129 genotype in human growth hormone-treated CJD patients in France and the UK. Lancet 2003; 362: 128-130.
- 19. Doh-ura K, Kitamoto T, Sakaki Y, Tateishi J. CJD discrepancy. Nature 1991; 353: 801-802.
- 20. Antoine JC, Michel D, Bertholon P, et al. Creutzfeldt-Jakob disease after extracranial dura mater embolization for a nasopharyngeal angiofibroma. Neurology 1997; 48: 1451-1453.
- 21. Dickinson AG, Fraser H, Outram GW. Scrapie incubation time can exceed natural lifespan. Nature 1975; 256: 732-733.
- 22. Hill AF, Joiner S, Linehan J, et al. Species-barrier-independent prion replication in apparently resistant species. Proc Natl Acad Sci USA 2000; 97: 10248-10253.
- 23. Frigg R, Klein MA, Hegyi I, et al. Scrapie pathogenesis in subclinically infected B-cell-deficient mice. J Virol 1999; 73: 9584-9588.
- 24. Race R, Chesebro B. Scrapie infectivity found in resistant species. Nature 1998; 392: 770.





Abstract
Although infectiousness is a feature of Creutzfeldt–Jakob disease (CJD), only a small proportion of cases are linked to transmission through healthcare provision.
As of January 2003, over 120 cases of CJD associated with use of human cadaveric dura mater had been recognised worldwide; almost all were associated with the commercial product Lyodura.
Most cases (97) have occurred in Japan, giving an overall risk estimate of around 1 per 2268 patients treated with Lyodura (0.04%) in that country.
In Australia, five cases of CJD have so far been linked to Lyodura, but, given the protracted tails of previous epidemics of transmissible spongiform encephalopathies, further cases are possible.
Results of surveys of Lyodura use in Australia are incomplete, but information from the manufacturer suggests that 2208–2478 sheets of Lyodura may have been used here.
This use translates to a relatively high incidence of Lyodura-associated CJD, with current overall rates appearing around five times higher than those reported in Japan; reasons for this difference are unclear.