Evidence-based medicine (EBM) is the current mantra in Australian healthcare. It also engages health policy deliberations, as the federal government has expressed its commitment to EBM’s essential values in making healthcare decisions. In 1998, in launching the Medicare Services Advisory Committee, the then Federal Minister for Health said:
. . . the introduction of evidence-based medicine and the committee means that the gap between research knowledge and clinical practice will narrow, and patients will benefit earlier from the most advanced procedures drawing on the best scientific and medical evidence.1
The Medicare Services Advisory Committee (now the Medical Services Advisory Committee), or MSAC, is the main national body responsible for assessing medical technology in Australia. Its role is to assess the safety, effectiveness and cost-effectiveness of new medical technologies and advise the government regarding their public funding. MSAC’s terms of reference and standard evaluation cycle are outlined in Box 1 and Box 2. It is portrayed as an independent, multidisciplinary scientific committee providing advice to the Minister, but at arm’s length from the political issues of policy implementation such as setting fees. MSAC has publicly signalled that its decisions will be underpinned by an evidence-based approach, based on rigorous, systematic reviews of the literature.4-6
In 1999, MSAC received applications from Wesley Hospital, in Brisbane, and the Peter MacCallum Cancer Institute, in Melbourne, seeking extension of Medicare funding for the use of positron emission tomography (PET). Until then, PET had had localised and limited public-funding arrangements at two quaternary-care centres in Sydney and Melbourne.7 Here we outline some aspects of the Commonwealth Review of Positron Emission Tomography (PETReview), which arose from these applications.
We believe that the PETReview process was flawed and that the report contains material errors of fact and evidence synthesis. Our analysis of the process calls into question the government’s commitment to the principles of EBM and the use of these principles in the generation of healthcare policy, including the deliberations of MSAC.
Much of the evidence substantiating our claims was obtained under the Freedom of Information Act 1982 (Cwlth).
An MSAC Executive teleconference initiated standard procedure to evaluate the first application from Wesley Hospital.8 But 9 days later, at a full committee meeting which the Health Minister attended as a visitor, MSAC changed direction. It was noted in the minutes that “the scope of indications for the diagnostic procedure are enormous”9 and “the economic issues surrounding PET are significant”.9 After discussion, the committee:
... agreed that the role of PET in the Australian health system needs to be clarified, and appropriate funding models considered, so that the application can be assessed in the broader context.9
MSAC’s chair subsequently wrote to the Minister seeking advice, indicating:
I am aware that you have some views on the issue. Given the implications PET has for the effective use of the health dollar from both a Commonwealth and a State/Territory perspective, I would appreciate your advice on this issue, which centres around appropriate management of the technology.10
In response to this request for advice, the Minister instructed the Australian Department of Health and Aged Care (now the Department of Health and Ageing [DoHA]) to set up the Review of Positron Emission Tomography, and asked MSAC to “contribute to the review by undertaking an assessment of the technology and its cost effectiveness”.11 During this time, the second application for extension of Medicare funding, from the Peter MacCallum Institute, was lodged, but both applications were deemed to have been subsumed by the PETReview process and were never formally assessed by MSAC or PETReview.
DoHA established a new committee (the Steering Committee), which had jurisdiction over broader policy issues and was charged with preparation of PETReview’s final report and formulation of recommendations to the Minister. The Steering Committee was chaired by MSAC’s deputy chair. Meanwhile, MSAC convened its Supporting Committee in standard fashion, with MSAC members, MSAC’s permanent medical adviser and co-opted clinical experts. MSAC nominated the NHMRC Clinical Trials Centre (CTC)12 as Contracted Evaluator. Its role was to identify the evidence, classify it according to the National Health and Medical Research Council (NHMRC) evidence taxonomy, and comment on the quality of the evidence (Box 2). The respective roles of the Steering Committee and Supporting Committee were clarified at the first Steering Committee meeting.13 MSAC prepared its own assessment report for the Minister, and this was bundled with the PETReview report.
Despite claims that the MSAC process is “open and transparent”,5 all the committee meetings were conducted in camera, and the minutes published on the DoHA website are an incomplete summary of the full minutes obtained under the Freedom of Information Act.
Box 3 contrasts the usual flow of information in MSAC assessments with PETReview’s information flow. The extent to which this review departed from MSAC’s usual protocol was subsequently clarified by MSAC’s chair:
While most overseas agencies set priorities for HTAs [health technology assessments], the agenda of MSAC has been determined by the flow of applications.
. . .
Because positron emission tomography (PET) has the potential to become a major expense for the Federal Government, MSAC recommended that the Minister establish a special committee (independent of the MSAC process, but with an MSAC member for coordination) to report on the need for this technology in Australia and its funding.4
Box 4 shows PETReview’s terms of reference and major findings.14 The primary finding, as identified by DoHA, was the conclusion that:
There is insufficient evidence at this time from which to draw definitive conclusions about the clinical and cost effectiveness of FDG [fluorodeoxyglucose] PET.15
We present some evidence to show why, in our opinion, this finding is not the highest-quality information available, was not produced through a rigorous, systematic review of the available literature, and is not a valid input for evidence-based decision-making.
Box 5 illustrates how, in our opinion, several elements of the PETReview process, such as multiple committees and time limitations, jeopardised the quality of the outcome.
We believe that the conclusion that there is insufficient evidence is invalid, because a large proportion of the evidence was not considered. This flaw is demonstrated by PETReview’s statement that “the indications reviewed account for about 40% of the clinical PET procedures conducted” (p. 27),14 and was compounded by the failure to consider all the evidence available for the indications reviewed.
We believe a second, crucial procedural flaw arose because of the distortion of information which occurred during transfer between the multiple committees. This introduced negative bias about the strength of the evidence for the effectiveness of PET. For example, despite acknowledging that a “key component of this review process was an assessment of PET conducted by a supporting committee of MSAC” (p. xiii),14 the pivotal finding of the Supporting (scientific) Committee that PET was “clinically effective” was altered by the Steering (policy) Committee to the finding that PET was “potentially clinically effective”.21 This change appears to have occurred without any documented authorisation by the Supporting Committee (the only committee which undertook detailed evaluation of evidence) or MSAC (which had ultimate responsibility for making decisions about the evidence).
In our opinion, PETReview departed from quality scientific method in several ways. These included misrepresentation of raw data and authors’ conclusions, inadequate response to critical comment, and refusal to retract acknowledged errors. Specific examples are detailed in Box 6.
The use of the report of the International Network of Agencies for Health Technology Assessment (INAHTA)22 by PETReview illustrates how we believe the advice of the Supporting Committee was overlooked. Published in 1999, this multinational report sought to document the global use of PET and to synthesise several PET technology assessments. However, several members of the Supporting Committee expressed reservations about the quality of this report.17 Some of the perceived problems were that the report included data for inferior forms of positron imaging, and that the method followed by INAHTA could not be classified as rigorous evidence-based medicine.31,32 A meeting of the Supporting Committee specifically noted that “there were too many inaccuracies” and the “Committee agreed not to include the INAHTA report in the CTC’s final report”.17 Despite this decision, the final reports of PETReview and MSAC make more than 30 references to the INAHTA report, including the statement “MSAC’s findings are largely consistent with the conclusions of existing reviews conducted by INAHTA” (p. xiii).14
Concerns about the use of the INAHTA evidence synthesis have been repeatedly brought to the attention of MSAC following the completion of the review,32,34 to little avail.
The Executive Summary of MSAC’s final PET Assessment Report states:
PET has improved diagnostic accuracy over conventional imaging in a number of indications. It has been shown to increase the detection of mediastinal and distant metastases not detected by conventional imaging in the staging of NSCLC [non-small-cell lung cancer]. It also has increased sensitivity in detecting metastatic disease in patients with melanoma or CRC [colorectal carcinoma] who are being considered for surgical resection.
. . .
There are documented examples where the results of PET have led to changes in patient management. An example is the avoidance of surgery in cancer patients with disseminated metastatic disease. If it is assumed that changes in management result in improvements in health outcomes, then it is reasonable to infer that improvements in diagnostic accuracy will lead to improved health outcomes. It is, however, not always clear how changed management will impact on clinical outcomes. It should be noted that the assumed relationship between diagnostic accuracy and health outcomes is not restricted to PET; the same assumptions will apply to most modern diagnostic technologies, because there is seldom information about their effects on health outcomes (pp. viii–ix).6
This summary demonstrates why the Supporting Committee’s authorised draft report concluded that PET is clinically effective in the indications reviewed,35 a view supported by invited external submissions to the review.14 The minutes of an MSAC meeting during PETReview’s evidence assessment include discussion about the benchmark to be used when evaluating diagnostic tests. MSAC’s medical adviser provided perspective when he stated that:
. . . the evidence of PET’s impact on clinical decision making and health outcomes is a key issue on which the advice of MSAC members is needed. There is no level 1 evidence on this aspect, but nor has there been for other diagnostic modalities, where MSAC has accepted the evidence supporting diagnostic accuracy and effect on decision making.36
It was noted that “the evidence for PET’s diagnostic accuracy is more robust than for CT/MRI so it becomes a matter of where the benchmark is set”.36
When MSAC deliberated on the final PET assessment report (May 2000), the committee’s medical adviser reminded members of the existing benchmarks when he recalled:
. . . at the previous MSAC meeting . . . it was agreed that evidence of diagnostic accuracy and of clinical utility was sufficient in principle for diagnostic tests, provided that there is evidence of effective treatment for the condition being tested for.37
MSAC’s medical adviser also cautioned that “To obtain evidence of a direct impact on health outcomes would require very large randomised trials, which are probably not feasible”.37
However, MSAC’s conclusion in the report, which it endorsed at that meeting, that “there is insufficient evidence on . . . clinical and cost effectiveness to warrant unrestricted Medicare Benefits Schedule funding” (p. ix),6 meant, in our opinion, that a new benchmark for the level of evidence required had effectively been set. It is relevant that the benchmark was reset with a clear appreciation that
. . . funding issues are the prerogative of the Commonwealth Review Steering Committee but that the objective was to retain funding at the current level.37
In our opinion, these new standards were not implemented through due process “easy for stakeholders to access and to understand”.5 Indeed, it appears that even MSAC’s own medical adviser was unsure of what standards were to be applied.
We have made our concerns about PETReview clear to representatives of MSAC and DoHA, and the Minister, providing them with our evidence. We asserted that the report required substantial revision, because the major findings would affect the care of individual patients, irrespective of the level of public funding support provided for the technology.
In our opinion, replies15,38 indicate that MSAC has not properly evaluated evidence and argument from us and from others,26,31,32 and, although errors of fact39 and omission27 have been acknowledged, no remediation has followed.
There appears to be little recourse for people who wish to see the public record in regard to PET put right. PETReview and MSAC were established by executive action and are not amenable to examination by the Commonwealth Ombudsman.40 The fact that MSAC has undertaken a review of the evidence means that other key members of the EBM establishment are reluctant to undertake further reviews.41 Individual committee members can provide little information about the decision-making process, as they are bound by permanent confidentiality agreements.15
A recent dictum of EBM is that “evidence doesn’t make decisions, people do”.42 No government has resources to fund all healthcare interventions that meet arbitrary benchmarks for clinical effectiveness and cost-effectiveness. Sensibly, the structures of MSAC and PETReview allowed the government to decide against implementing recommendations without qualification.
However, we believe that the government has inappropriately chosen to represent its decisions as a logical consequence of independent scientific advice, and has harnessed the power of EBM to suppress dissent. The process by which many Australians have been denied access to PET falls far short of the promise made at MSAC’s launch that:
. . . the gap between research knowledge and clinical practice will narrow, and patients will benefit earlier from the most advanced procedures drawing on the best scientific and medical evidence.1
We believe this situation can only be remedied by a review of MSAC’s final assessment report on PET, with procedures to ensure that MSAC’s process and resources are sufficient to meet its stated quality objectives. Steps are also required to ensure that the independence of MSAC is guaranteed by legislation, so that the committee can pursue its important task without political intervention.
1: Medical Services Advisory Committee terms of reference2
MSAC’s terms of reference are to:
advise the Minister for Health and Ageing on the strength of evidence pertaining to new and emerging medical technologies and procedures in relation to their safety, effectiveness and cost-effectiveness and under what circumstances public funding should be supported;
advise the Minister on which new medical technologies and procedures should be funded on an interim basis to allow collection of data to determine their safety, effectiveness and cost-effectiveness;
advise the Minister on references related either to new or existing medical technologies and procedures; and
undertake health technology assessment work referred by the Australian Health Ministers’ Advisory Council (AHMAC), and report its findings to AHMAC.
2: The Medical Services Advisory Committee (MSAC) assessment process3
1. Eligibility assessment
2. Assessment
MSAC reviews are undertaken by supporting committees. Supporting committees are chaired by a member of MSAC and comprise people with relevant expertise nominated by medical colleges, and others with expertise in economics, epidemiology or consumer issues as required. Draft assessment reports are developed by the contractors, but the supporting committees are responsible for the issues covered and formulating draft recommendations for MSAC.
3. Advice to the Minister
Based on the draft assessment reports and recommendations, MSAC formulates its advice and recommendations to the Minister.
These recommendations generally fall into one of three categories:
that funding should be supported;
that funding should not be supported; or
that funding should be provided on an interim basis to enable further data collection.
4. Decision
5. Implementation
3 Information flow in standard Medical Services Advisory Committee (MSAC) assessments, and in PETReview
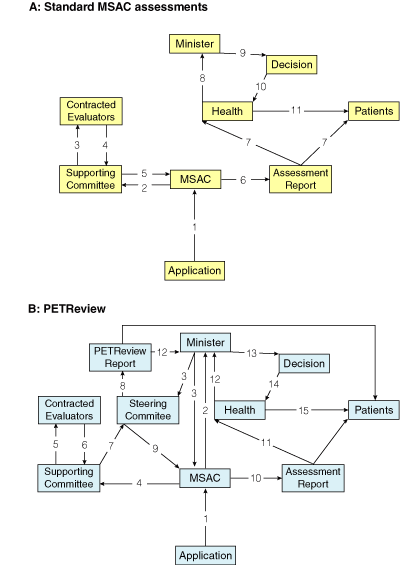
Numbers indicate the order in which information travels to the various participants.
4: Terms of reference for PETReview and summary of major findings*14
Terms of reference |
Major findings |
||||||||||
1 To assess the cost-effectiveness, clinical effectiveness and safety of positron emission tomography (PET), especially in relation to other diagnostic modalities. |
|
||||||||||
2 To report on and assess the state of PET technology, recommending preferred technical specifications and approaches where appropriate. |
|
||||||||||
3 To clarify the role of PET in Australian clinical practice, including: |
|
||||||||||
3.1 determining which indications/applications should be eligible for funding; and |
|
||||||||||
3.2 where funding is appropriate, determining suitable funding models. |
|
||||||||||
4 To develop a national strategy aimed at ensuring appropriate distribution of and access to PET services. |
|
||||||||||
5 To develop a data collection and analysis plan to enable the ongoing evaluation of PET. |
|
||||||||||
* Indications reviewed were evaluation of solitary pulmonary nodules, primary staging of non-small-cell lung cancer, evaluation of potentially resectable metastatic melanoma/recurrent colorectal carcinoma, evaluation of residual/recurrent mass in patients with malignant glioma/colorectal carcinoma, primary staging of malignant glioma, and assessment of medically refractory epilepsy/myocardial viability in patients being considered for surgery. |
|||||||||||
5: Quality limitations of PETReview resulting directly from the process adopted
Limited resources prejudiced the scope and quality of PETReview
Medical Services Advisory Committee (MSAC) assessments are not time-limited, but PETReview had a 6-month time frame. More than 2 months was used in constituting committees. When work began, it was clear that insufficient time and resources had been allocated to meet the terms of reference. It was later admitted that:
Given the time frame of the broader review, it was not possible for MSAC to thoroughly review the evidence for PET in all the clinical scenarios in which it is used.16
Supporting Committee minutes indicate quality was impaired because of resource constraints:
One of the difficulties that the CTC [NHMRC Clinical Trials Centre] had in addressing concerns raised by the committee was that different areas of the report had been done by different people and that they had had limited time.
. . .
CTC did not have a copy of the minutes; so they were working based on various discussions they had with people after the meeting and, for example, [the involved individual] was making amendments based on second hand notes.17
Limited resources precluded adequate peer review of PETReview
Although it was planned that they should do so, the Supporting Committee and the 49 stakeholders who made submissions14 did not view (and thus were not able to comment on) the final report of MSAC and PETReview until after the Minister had accepted the recommendations.
Poor organisation prejudiced the integrity of PETReview
MSAC usually has at most two committees working on assessments, but four committees (supporting, steering, technical, MSAC) contributed to PETReview, and these committees apparently did not communicate adequately, even though some members belonged to more than one committee. There is no record of direct contact taking place, or minutes being exchanged.
Non-standard operating guidelines prejudiced PETReview decision-making
Standard MSAC Supporting Committee procedures require all dissent from draft report findings to be explicitly documented. This requirement was omitted from guidelines given to PETReview Supporting Committee participants, and it appears that their concerns (documented in minutes of the meetings17-19) were not communicated to MSAC.
The report of the contracted evaluators was given undue influence
MSAC standard evaluation guidelines stipulate that co-opted clinical experts must decide the clinical relevance of evidence gathered and summarised by the contracted evaluators. The contracted evaluators for PETReview were given much greater authority for PETReview by the chair when addressing the Steering Committee: “. . . findings of the CTC evaluation were key to the outcome of the review”.20 While several of the clinicians on the Supporting Committee raised concerns about some aspects of the CTC report, “the Chair reminded the committee that the CTC report was a report on the evidence, and that it was the supporting committee’s role to make recommendations to MSAC based on that evidence”.17
6: Flaws in PETReview’s scientific process
Inclusion of evidence of limited quality
As an example, inclusion of the International Network of Agencies for Health Technology Assessment (INAHTA) report22 (see text page 628).
Omission of high level evidence
A meta-analysis by Dwamena et al,23 published at the same time as the INAHTA report, found that positron emission tomography (PET) had significantly greater diagnostic accuracy than computed tomography for mediastinal staging of non-small-cell lung cancer, but this study was not considered by PETReview.
Misinterpretation of data and authors’ conclusions
The PETReview report makes three comments about an article by Flamen et al24 relating to the use of PET in colorectal cancer, including confirmation that the evidence quality was good.
A comment by the Medicare Advisory Services Committee (MSAC) that “Discordance occurred in only 16% of locoregional recurrences and 10% of investigations elsewhere” accurately reflects the data, but omits the more clinically relevant perspective that these discordances resulted in PET providing significant incremental diagnostic value in 20% to 60% of patients, depending upon the clinical indication.
The report’s comment that “Flamen et al also made the important point that, in almost all of the patients for whom PET was superior to other methods, conventional diagnostic methods had given equivocal results rather than negative or positive results” (p. 54)6 is not accurate. Flamen has confirmed that no such emphasis or similar statement can be found in his article,25 primarily because the published data could not support such a conclusion. Only 5 of 21 patients in whom PET improved diagnosis were part of the subgroup with equivocal findings.
Negative bias is reinforced because the article’s conclusion is omitted from the report: “Whole-body FDG-PET can have a clear impact on the therapeutic management in the follow-up of patients with colorectal cancer”.
Kalff expressed concerns to MSAC that PETReview had misrepresented his data.26 A representative of the CTC acknowledged:
An additional comment in this section on the value of demonstrating metastatic disease from your paper would have been appropriate, and we accept that this is an omission, but not one which alters the final recommendations of the report.27
Kalff’s prospective series of 105 lung cancer patients showed management changes in 67%, almost all validated as appropriate, providing good evidence of the ability of PET to improve patient outcomes in this context.
PETReview states:
Furthermore, the Wesley Hospital submission, which demonstrated cost savings in an Australian setting for NSCLC [non-small-cell lung cancer] based on the Gambhir et al (1996) model, is likely to be inaccurate (p. 81).6
MSAC’s decisions about Australian cost-effectiveness data are critically dependent on its assessment that Gambhir’s data28 are of lower quality and resulted in a different conclusion to a subsequent article by Scott et al.29 Gambhir, who co-authored both articles, has indicated that MSAC’s representation of these articles contained significant errors of fact.30
Inadequate response to critical comment
Several PETReview participants noted errors of fact and interpretation during the Supporting Committee evidence evaluation,18,19 but corrections were incomplete.
Several correspondents, including a Supporting Committee member,31-33 argued that PETReview and MSAC’s final reports contained many errors of fact and interpretation, and that the report was unfairly biased. These critiques have not been fully addressed by MSAC, a failure justified by a representative of the Department of Health and Ageing as “given MSAC’s already considerable workload, the volume and complexity of your correspondence militated against its being tabled in its entirety”.15
No revision of MSAC’s or PETReview’s report has been made in response to critical comment about the evaluation of evidence.
- Robert E Ware1
- Hilton W Francis2
- Kenneth E Read3
- 1 49 Augusta Road, Lenah Valley, TAS.
- 2 4 Warneford Street, South Hobart, TAS.
- 3 Malthouse Chambers, Battery Point, TAS.
We thank Dr Rodney Hicks and Dr Victor Kalff for editorial assistance and encouragement.
R E Ware acknowledges that increased use of PET may result in personal financial gain. The author owns a PET camera, and has shares in a private biotechnology company which supplies radioisotopes. He also supplies medical services to MIA Group Pty Ltd. K E Read and H W Francis have no competing interests.
- 1. Wooldridge M. Australia first in world to adopt evidence based medicine. Media release MW 77/98. 6 Apr 1998. Available at: www.health.gov.au/archive/mediarel/1998/mw7798.htm (accessed May 2004).
- 2. Medical Services Advisory Committee. Terms of reference. Available at: www.msac.gov.au/terms.htm (accessed May 2004).
- 3. Medical Services Advisory Committee. Newsletter. June 2001. Available at: www.msac.gov.au/newsletter.htm (accessed May 2004).
- 4. Weedon D. Health technology assessment in Australia. Med J Aust 1999; 171: 551-552.
- 5. Weedon D. Official launch of MSAC [speech]. 6 Apr 1998. Available at: www.health.gov.au/msac/chair.htm (accessed May 2004).
- 6. Medicare Services Advisory Committee. Positron emission tomography. MSAC assessment report. Canberra: MSAC, 2000. Available at: www.health.gov.au/haf/pet/petfinal.htm (accessed May 2004).
- 7. Morris JG. The stage is set for the diffusion of positron emission tomography (PET) in oncology. Med J Aust 1999; 171: 527-528. <MJA full text>
- 8. Medicare Services Advisory Committee. Minutes of executive committee teleconference, 10 May 1999. FOI release.
- 9. Medicare Services Advisory Committee. Minutes of meeting, 19 May 1999. FOI release.
- 10. Weedon D (Chair, Medicare Services Advisory Committee). Letter to M Wooldridge, Minister for Health and Aged Care. Jun 1999. FOI release.
- 11. Wooldridge M (Minister for Health and Aged Care). Letter to D Weedon, Chair, Medicare Services Advisory Committee. Aug 1999. FOI release.
- 12. Medicare Services Advisory Committee. Minutes of executive committee meeting, 8 Sep 1999. FOI release.
- 13. Commonwealth Review of Positron Emission Tomography. Minutes of Steering Committee meeting, 13 Oct 1999. FOI release.
- 14. Report of the Review of Positron Emission Tomography. Canberra: Commonwealth of Australia, 2000. Available at: www.health.gov.au/haf/pet/petfinal.htm (accessed May 2004).
- 15. Halton J (Secretary, Department of Health and Ageing). Letter to R Ware. 2 Apr 2002.
- 16. Weedon D (Chair, Medicare Services Advisory Committee). Letter to R Ware. 22 Sep 2000.
- 17. Commonwealth Review of Positron Emission Tomography. Minutes of Supporting Committee meeting, 28 Feb 2000. FOI release.
- 18. Hicks R. Comments on the NHMRC Trial Centre review of PET literature. Attachment A in Commonwealth Review of Positron Emission Tomography. Minutes of Supporting Committee meeting, 14 Feb 2000. FOI release.
- 19. Miles K. A response to the draft report of NHMRC Clinical Trials Centre. Attachment B in Commonwealth Review of Positron Emission Tomography. Minutes of Supporting Committee meeting, 14 Feb 2000. FOI release.
- 20. Commonwealth Review of Positron Emission Tomography. Minutes of Steering Committee meeting, 27 Jan 2000. FOI release.
- 21. Commonwealth Review of Positron Emission Tomography. Minutes of Steering Committee meeting, 6 Apr 2000. FOI release.
- 22. Adams E, Asua J, Olasagasti JC, et al. Positron emission tomography: experience with PET and synthesis of the evidence. Stockholm: INAHTA, 1999. Available at: www.inahta.org (accessed May 2004).
- 23. Dwamena B, Sonnad S, Angobaldo J, Wahl R. Metastases from non-small cell lung cancer: mediastinal staging in the 1990s — meta-analytic comparison of PET and CT. Radiology 1999; 213: 530-536.
- 24. Flamen P, Stroobants S, Van Cutsem E, et al. Additional value of whole-body positron emission tomography with fluorine-18-2-fluoro-2-deoxy-d-glucose in recurrent colorectal cancer. J Clin Oncol 1999; 17: 894-901.
- 25. Flamen P (Head, Department of Nuclear Medicine, Bordet, Brussels). Letter to R Ware. 24 May 2001.
- 26. Kalff V (Associate Professor of Medicine, Alfred Hospital, Prahran, VIC). Letter to D Weedon, Chairman, MSAC PET review committee. 29 Jan 2001.
- 27. Simes J (Director, NHMRC Clinical Trials Centre). Letter to V Kalff. 13 Mar 2001.
- 28. Gambhir SS, Hoh CK, Phelps ME, et al. Decision tree sensitivity analysis for cost-effectiveness of FDG-PET in the staging and management of non-small-cell lung carcinoma. J Nucl Med 1996; 37: 1428-1436.
- 29. Scott WJ, Shepherd J, Gambhir SS. Cost-effectiveness of FDG-PET for staging non-small cell lung cancer: a decision analysis. Ann Thorac Surg 1998; 66: 1876-1883.
- 30. Gambhir SS (Director, Crump Institute for Molecular Imaging, UCLA School of Medicine, Los Angeles, Calif.). Letter to R Ware. 27 Aug 2002.
- 31. Hicks R (Director, Diagnostic Imaging, Peter MacCallum Cancer Institute). Letter to D Weedon, Medicare Services Advisory Committee. 5 Jan 2001.
- 32. Hicks R (Director, Diagnostic Imaging, Peter MacCallum Cancer Institute). Letter to R King, Medicare Services Advisory Committee. 7 Mar 2001.
- 33. Ware R. Letter to D Weedon, Chair, Medicare Services Advisory Committee. 20 Mar 2001.
- 34. Ware R. Letter to M Wooldridge, Minister for Health and Aged Care. 31 Jan 2001.
- 35. Commonwealth Review of Positron Emission Tomography. Draft MSAC Supporting Committee report. Appended to minutes of Supporting Committee meeting, 23 Mar 2000. FOI release.
- 36. Medicare Services Advisory Committee. Minutes of meeting, 23 Feb 2000. FOI release.
- 37. Medicare Services Advisory Committee. Minutes of meeting, 24 May 2000. FOI release.
- 38. Keith A (Assistant Secretary, Diagnostics and Technolgoy Branch, Department of Health and Aged Care). Letter to R Ware. 1 Aug 2001.
- 39. Simes J (Director, NHMRC Clinical Trials Centre), Irwig L (Professor of Epidemiology, University of Sydney), Coleman K (Project Manager, NHMRC Clinical Trials Centre). Letter to J Hastings, Department of Health and Aged Care. 13 Aug 2002.
- 40. Murphy G (Senior Investigation Officer, Commonwealth Ombudsman). Letter to R Ware. 14 May 2002.
- 41. Pettigrew A (Chief Executive Officer, NHMRC). Letter to R Ware. 7 May 2001.
- 42. Haynes RB, Devereaux PJ, Guyatt GH. Physicians’ and patients’ choices in evidence based practice. BMJ 2002; 324: 1350.





Abstract
The Commonwealth Government constituted the Medicare Services Advisory Committee (MSAC) to implement its commitment to entrench the principles of evidence-based medicine in Australian clinical practice.
With its recent review of positron emission tomography (PETReview), the Commonwealth intervened in an established MSAC process, and sanctioned the stated objective to restrict expenditure on the technology.
In our opinion:
The evaluation of evidence by PETReview was fundamentally compromised by a failure to meet the terms of reference, poor science, poor process and unique decision-making benchmarks.
By accepting the recommendations of PETReview, the Commonwealth is propagating information which is not of the highest quality.
The use of inferior-quality information for decision-making by doctors, patients and policy-makers is likely to harm rather than enhance healthcare outcomes.