Despite widespread public acknowledgement of its dangers, tobacco smoking remains the single greatest risk factor for disease burden in Australia today.1
Most people who try to stop smoking do so unassisted,2 and many return to smoking within a few months.3 There is now substantial evidence that pharmacotherapy, such as nicotine replacement therapy (NRT), can significantly increase an individual’s chances of stopping.4 Indeed, it is widely recommended that pharmacotherapy be incorporated into any quit attempt when not contraindicated.5
More recently, psychological and behavioural strategies are being recommended as an adjunct to pharmacotherapy for smoking cessation.5-8 Telephone counselling has great potential as adjunctive therapy, as it is easily accessible and flexible, and eliminates the need for face-to-face contact, so is particularly beneficial for those in remote areas or with busy schedules, and those reluctant to engage in face-to-face counselling because of perceived stigma. However, research on telephone counselling for smoking cessation has had varied results.9 This may be partly due to the range of smoking-cessation products used and diversity in the design of telephone-counselling trials.
Our study examined the efficacy of a telephone-counselling program as an adjunct to NRT. The program involved proactive telephone counselling with a relapse-sensitive call schedule (ie, more calls were made in the early stages of the quit attempt, when the risk of relapse is greatest10). This type of call schedule has been shown to reduce relapse rates.11,12 The service also had a reactive component, with participants invited to telephone a counsellor when necessary.
The study was a randomised controlled trial and was approved by the Human Research Ethics Committee, University of Sydney, New South Wales. It was conducted between October 2001 and August 2002.
Brief (5-minute) follow-up telephone questionnaires were administered to all participants at 1, 2, 3 and 6 months after baseline by non-counselling data collectors at the GlaxoSmithKline call centre. Participants were asked about dates of patch use (at the 1-, 2- and 3-month calls) and whether they had smoked since the previous call (all calls); if so, they were asked the number of cigarettes smoked per day and the date of the last cigarette smoked in any renewed quit attempts. To minimise misleading reports of abstinence, a bogus pipeline technique was used,13 with the possibility of carbon monoxide breath testing mentioned in the consent form and at the 3- and 6-month monitoring calls.
The counselling group was also asked about the perceived benefits of counselling. Data collectors were therefore not blinded to participant allocation.
Participants were paid $20 per follow-up call, independent of their smoking status. This was to increase cooperation and was considered adequate reward for the time involved, but not sufficient to induce participation solely for financial gain.
The outcome of primary interest was smoking abstinence, assessed as:
28-day continuous abstinence at each of the 3- and 6-month calls, defined as complete abstinence (“not even a puff”) for at least the previous 28 days; and
90-day continuous abstinence at the 6-month call (ie, the reported date of the last cigarette smoked was at least 90 days before the 6-month follow-up call).
The latter is a more realistic indicator of long-term effectiveness, as it allows for the dynamic nature of quit attempts, where smokers typically lapse or relapse in the early stages.14
A secondary outcome was the number of weeks use of NRT and its relationship to success in the quit attempt.
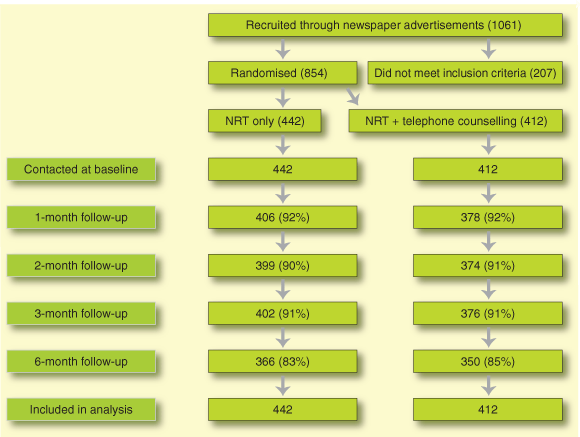
A total of 1061 smokers responded to advertisements, but 207 did not meet inclusion criteria (175 on medical grounds and 32 for other reasons, such as moving interstate or smoking less than 10 cigarettes per day). The remaining 854 were randomly allocated (Box 1).
Baseline demographic characteristics and smoking history of the 854 participants are shown in Box 2. Participants were representative of the wider Australian population of smokers15 and had typically made several quit attempts either unassisted or using a variety of aids.
Abstinence rates are shown in Box 3. Participants who received telephone counselling were significantly more likely to achieve 28-day abstinence at both 3 and 6 months than those using NRT alone (difference at 3 months, 6.5%, P = 0.04; difference at 6 months, 7.7%, P = 0.01). Those who received counselling were also significantly more likely to achieve long-term abstinence, with higher odds of 90 days’ abstinence at 6 months (difference, 8.1%; P = 0.004).
This study demonstrated that telephone counselling can significantly increase the likelihood of achieving long-term abstinence from smoking when used as an adjunct to NRT. These results were obtained under conditions that closely mirror self-initiated attempts to quit smoking using over-the-counter nicotine patches.
The rates of abstinence among those who received NRT alone in our study compared favourably with rates reported in reviews of use of over-the-counter NRT.16 This further supports the efficacy of over-the-counter NRT.
To date, four randomised trials have specifically compared quit rates of smokers receiving NRT with and without telephone support,17-20 but none reported a significant benefit of telephone support beyond 3 months. Two important features differentiate our program from the programs in these studies: use of a relapse-sensitive call schedule (used in only one previous study17), and delivery by highly trained and experienced counsellors (with more experience than in at least two previous studies17,18).
The extent to which the written self-help material contributed to abstinence is difficult to determine, as it was not tested in isolation from telephone counselling. There is some evidence that such material may increase abstinence rates for those receiving no other assistance or receiving behavioural support alone, but it has not been shown to contribute significantly to abstinence rates in those using NRT.21
A limitation of our study was the absence of biochemical verification of abstinence, as this was not logistically feasible in a study this large. Furthermore, current methods of biochemical verification cannot reliably verify abstinence from smoking beyond 7 days,14 reducing their usefulness for verifying longer-term abstinence. In addition, high levels of sensitivity and specificity are regularly obtained with self-report measures.22
This randomised controlled trial provided evidence under “real world” conditions that telephone counselling as an adjunct to NRT increases longer-term abstinence rates beyond those achieved with NRT alone. Thus, referral to and support for smoking-cessation telephone-counselling services should be encouraged.
2: Baseline characteristics of the 854 study participants
|
NRT only (n = 442) |
NRT + counselling (n = 412) |
|||||||||
No. of men |
226 (51%) |
191 (46%) |
|||||||||
Mean age (years) (95% CI) |
40.9 (39.8–42.0) |
42.6 (41.6–43.6) |
|||||||||
Mean cigarettes per day (95% CI) |
24.2 (23.3–25.1) |
24.0 (23.0–24.9) |
|||||||||
Mean age started smoking (95% CI) |
16.8 (16.3–17.2) |
16.6 (16.1–17.1) |
|||||||||
Mean number of quit attempts (95% CI) |
2.9 (2.6–3.1) |
2.7 (2.5–3.0) |
|||||||||
No. with previous use of NRT patches |
121 (27%) |
121 (29%) |
|||||||||
No. with previous use of NRT gum |
127 (29%) |
107 (26%) |
|||||||||
No. with previous attempts to quit unassisted |
321 (73%) |
303 (74%) |
|||||||||
NRT = nicotine replacement therapy. |
|||||||||||
3: Rates of smoking abstinence according to treatment group
Outcome |
NRT only (n = 442) |
NRT + counselling (n = 412) |
Odds ratio (95% CI) |
||||||||
28-day abstinence at 3 months |
25.1% |
31.6% |
1.38 (1.02–1.85) |
||||||||
28-day abstinence at 6 months |
22.4% |
30.1% |
1.49 (1.10–2.03) |
||||||||
90-day abstinence at 6 months |
18.6% |
26.7% |
1.60 (1.16–2.21) |
||||||||
NRT = nicotine replacement therapy. |
|||||||||||
- Zane R Macleod1
- Veronica C Arnaldi2
- Ian M Adams3
- Margaret A Charles4
- 1 Medical and Scientific Affairs, GlaxoSmithKline Consumer Healthcare, Sydney, NSW.
- 2 School of Psychology, University of Sydney, Sydney, NSW.
We thank the following people: Alan Moore, Betty Jago, Evelyn Stevenson, Jackie Carey, Jason Vella, John Harris, Kimberley O'Brien, and Richard Erber (NicabateCQ counsellors); Sonya Kraus and Eleanor Spence for conducting the monitoring calls; and Professor Saul Shiffman, Dr Ron Borland and Dr Fiona Dunagan for their helpful comments. We thank GlaxoSmithKline Consumer Healthcare, which supplied the NicabateCQ transdermal nicotine patches; and the services of the NicabateCQ Committed Quitters telephone counselling program.
Mr Zane Macleod and Dr Margaret Charles were contracted by GlaxoSmithKline Consumer Healthcare and remunerated for their work on this research. Before and during the conduct of this research, Zane Macleod was contracted by GlaxoSmithKline Consumer Healthcare as principal counsellor of the NicabateCQ Committed Quitters Programme. Veronica Arnaldi and Ian Adams were employees of GlaxoSmithKline Consumer Healthcare and were responsible for the operational management of the research. Remuneration of Ian Adams by GlaxoSmithKline included stock options. This study was funded by GlaxoSmithKline Consumer Healthcare. The conduct of the study was independently monitored and the data verified by Datapharm Australia. GlaxoSmithKline took part in discussions about study design, but had no direct role in the analysis or interpretation of the results or preparation of the report for publication; these were undertaken by Zane Macleod and Margaret Charles.
- 1. Mathers CD, Vos ET, Stevenson CE, Begg SJ. The burden of disease and injury in Australia. Bull World Health Organ 2001; 79: 1076-1084.
- 2. Borland R. Promoting cessation of tobacco use. In: Scollo M, Chapman S, editors. Tobacco control in Australia: a priority-driven research agenda. Sydney: National Heart Foundation of Australia and Australian Cancer Society, 1999.
- 3. Hughes JR, Gulliver SB, Fenwick JW, et al. Smoking cessation among self-quitters. Health Psychol 1992; 11: 331-334.
- 4. Silagy C, Lancaster T, Stead L, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2002; 4: CD000146.
- 5. The Smoking Cessation Clinical Practice Guideline Panel and Staff and Consortium Representatives. A clinical practice guideline for treating tobacco use and dependence: a US Public Health Service Report. JAMA 2000; 283: 3244-3254.
- 6. Hughes JR, Fiester S, Goldstein M, et al. Practice guideline for the treatment of patients with nicotine dependence. American Psychiatric Association. Am J Psychiatry 1996; 153 (10 Suppl): S1-S31.
- 7. Smoking Cessation Guideline Panel. Clinical practice guideline 18: smoking cessation. Washington, DC: Agency for Health Care Policy Research, 1996.
- 8. National Prescribing Service. Prescribing practice review: smoking cessation. Sydney: National Prescribing Service Limited, 2002.
- 9. Stead L, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2002; (4).
- 10. Zhu SH, Tedeschi GJ, Anderson CM, Pierce JP. Telephone counselling for smoking cessation: what's in a call? J Couns Dev 1996; 75: 93-102.
- 11. Zhu SH, Stretch V, Balabanis M, et al. Telephone counseling for smoking cessation: effects of single-session and multiple session interventions. J Consult Clin Psychol 1996; 64: 202-211.
- 12. Borland R, Segan CJ, Livingston PM, Owen N. The effectiveness of callback counselling for smoking cessation: a randomised trial. Addiction 2001; 96: 881-889.
- 13. Jones E, Sigall H. The bogus pipeline: a new paradigm for measuring affect and attitude. Psychol Bull 1971; 76: 349-364.
- 14. Velicer WF, Rochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull 1992; 111: 23-41.
- 15. Adhikari P, Summerill A. 1998 National Drug Strategy household survey: detailed findings. Drug statistics series no. 6. Canberra: Department of Health and Ageing, 2000.(AIHW cat. no. PHE-27.)
- 16. Hughes JR, Shiffman S, Callas P, et al. A meta-analysis of the efficacy of over-the-counter nicotine replacement. Tob Control 2003; 12: 21-27.
- 17. Solomon LJ, Scharoun GM, Flynn BS, et al. Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Prev Med 2000; 31: 68-74.
- 18. Ockene JK, Kristeller J, Goldberg R, et al. Increasing the efficacy of physician-delivered smoking interventions: a randomized clinical trial. J Gen Intern Med 1991; 6: 1-8.
- 19. Lando HA, Rolnick S, Klevan D, et al. Telephone support as an adjunct to transdermal nicotine in smoking cessation. Am J Public Health 1997; 87: 1670-1674.
- 20. Reid RD, Pipe A, Dafoe A. Is telephone counselling a useful addition to physician advice and nicotine replacement therapy in helping patients to stop smoking? A randomized controlled trial. CMAJ 1999; 160: 1577-1581.
- 21. Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2002; 3: CD001118.
- 22. Patrick D, Cheadle A, Thompson D, et al. The validity of self-reported smoking: a review and meta-analysis. Am J Public Health 1994; 84: 1086-1093.





Abstract
Objectives: To investigate the effectiveness of telephone counselling as an adjunct to nicotine replacement therapy (NRT) by transdermal patch in smoking cessation.
Design: Randomised controlled trial.
Participants and setting: 854 smokers from New South Wales, aged 18 years and older, who had smoked at least 10 cigarettes per day for the past year and responded to newspaper advertisements between October 2001 and January 2002; the trial was conducted between October 2001 and August 2002.
Interventions: Random allocation to either NRT alone or NRT plus telephone counselling (5 sessions spaced according to a relapse-sensitive call schedule).
Main outcome measures: Self-reported abstinence assessed by telephone questionnaires at 1, 2, 3 and 6 months: 28-day continuous abstinence at 3 and 6 months, and 90-day continuous abstinence at 6 months.
Results: 28-day continuous abstinence rates among participants receiving telephone counselling were significantly greater than among those not receiving telephone counselling at both 3 and 6 months (31.6% v 25.1%; P = 0.04 at 3 months; and 30.1% v 22.4%; P = 0.01 at 6 months). Similarly, 90-day continuous abstinence rates at 6 months were significantly greater for participants receiving counselling (26.7% v 18.6%; P = 0.004).
Conclusion: Telephone counselling as an adjunct to NRT increases abstinence rates beyond the use of NRT alone.