Australia has been considered to be an iodine-replete country, with only isolated pockets of mild to moderate iodine deficiency. Evidence from recent studies suggests that this assumption may no longer be valid.1-4
If physiological iodine requirements are not met, abnormalities of thyroid development and function occur (Box 1). Following studies in Sydney,1-3 it was hypothesised that iodine deficiency in Australia may be increasing and causing goitre in urban populations.22,23
A pilot study by the Department of Endocrinology and Diabetes at our hospital between October 1999 and August 2000 examined a population of children referred with goitre: 81% (26/32) were found to have urinary iodine levels < 100 μg/L; 13/16 patients who subsequently had thyroid technetium scanning had increased uptake in the range of 8%–20% (normal range, 2%–5%), and 25/26 iodine-deficient patients were thyroid antibody negative and had normal levels of thyroid-stimulating hormone (TSH).24 This constellation of antibody-negative goitre with high uptake on scanning suggests iodine deficiency or congenital thyroid dyshormonogenesis as the cause. As the latter is rare, iodine deficiency is the most likely cause of goitre in this group.
We report here a study aimed at examining iodine status and prevalence of goitre in a larger sample of schoolchildren to estimate iodine status within the Melbourne community.
We assessed two clinical indicators of thyroid disease: goitre (by thyroid gland palpation) and urinary iodine levels.
Two qualified endocrinologists attended each school and performed thyroid palpation according to World Health Organization recommendations, with three grades used for classification of thyroid size: 25
Grade 0: No palpable or visible goitre;
Grade 1: Palpable but not visible goitre;
Grade 2: Palpable and visible goitre.
Urine samples were taken about two hours after rising in all subjects. All samples for urinary iodine analysis (identified by code only) were sent to the Institute of Clinical Pathology and Medical Research at Westmead Hospital, Sydney. Urinary iodine levels were measured by an assay based on the Sandell–Kolthoff reaction. We categorised urinary iodine values according to level of deficiency: 25
< 20 μg/L, severe iodine deficiency;
20–49 μg/L, moderate iodine deficiency;
50–99 μg/L, mild iodine deficiency; and
≥ 100 μg/L, iodine-replete status.
Six hundred and seven students aged 11–18 years from the two schools gave their informed consent, and 410 girls (71%) and 167 boys (29%) were included in the study. The remaining 30 schoolchildren were unable to provide a urine sample on the day of the study. The girls were from Years 5–12, and the boys were from Years 7–12. Participation rates were greater at the girls school (50%) than the boys school (14%).
Median urinary iodine excretion for the total population was 70 μg/L. Median urinary iodine excretion was calculated for boys (82 μg/L), girls (64 μg/L), school year and goitre status (Box 2). No median exceeded the 100 μg/L level recommended by WHO as indicative of iodine sufficiency in a population.
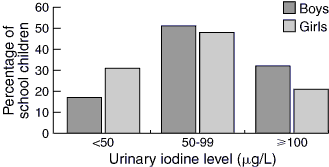
The association of moderate–severe (< 50 μg/L) and mild (50–99 μg/L) iodine deficiency and sex, school year and presence of goitre was determined. There were significantly more girls with urinary iodine levels < 50 μg/L (P < 0.001), and this significant difference remained (P = 0.002) for urinary iodine values < 100 μg/L (Box 3).
There was no association between goitre grade and moderate–severe (P = 0.39) or mild (P = 0.07) iodine deficiency (Box 4), but we noted an association, independent of sex, between school year and moderate iodine deficiency (P = 0.03), but not for mild deficiency (P = 0.27).
Use of logistic regression showed that the odds of a girl having iodine deficiency (urinary iodine level < 50 μg/L) was 2.5 times greater (95% CI, 1.57–4.12) than for a boy, adjusted for school year. Repeat of this analysis for urinary iodine values < 100 μg/L gave odds of 1.9 (95% CI, 1.2–3.0) for girls compared with boys when adjusted for school year.
Logistic regression analysis was used to examine whether variations in sex distribution, and therefore iodine levels, in the schoolchildren in different school years may have masked a true effect of iodine level variation in different age groups. The results of this analysis are shown in Box 5 for all urinary iodine values < 50 μg/L. A significant difference was demonstrated for Years 7 and 12 and a marginally significant difference for Years 8, 10 and 11 using Year 5 as a reference point once school year was adjusted for sex. A similar analysis was undertaken for urinary iodine values < 100 μg/L, but no difference between years was detected.
The results of our study substantiate growing concerns that dietary iodine deficiency is increasing in prevalence in Australia. We need to know whether the recent influx of studies demonstrating iodine deficiency1-4 indicates a re-emergence, or an ongoing problem, of iodine deficiency.
Our study design is based on WHO recommendations that at least two modes of surveillance should be used and a large study sample targeted.25 Urinary iodine levels reflect dietary iodine intake, as 90% of ingested iodine is eventually excreted in the urine. WHO guidelines recommend use of spot urinary specimens to provide an adequate assessment of a population's iodine status.25 Thyroid gland palpation is a quick, easy and acceptable method to assess the presence of goitre. All subjects were assessed by two trained clinicians to reduce the incidence of interobserver bias, which has been reported in previous studies to affect 16%–40% of assessments.26,27
Thyroid ultrasound scanning, which is considered to be a much more useful tool to assess thyroid volume, especially in areas of mild iodine deficiency,26 was not available for use in our study.
Studies of schoolchildren reflect the current iodine status of a given community, and have the extra advantage that the children can be followed up to assess the efficacy of introduced control measures.25
The median urinary iodine excretion level of 70 μg/L (64 μg/L for girls and 82 μg/L for boys) is below the accepted criterion of 100 μg/L set by WHO. An iodine-replete population is indicated by at least 50% of the population achieving a urinary iodine value ≥ 100 μg/L and less than 20% having a urinary iodine level < 50 μg/L.25,28 Only 24% of our population had a urinary iodine level ≥ 100 μg/L and 27% had a urinary iodine level < 50 μg/L. Despite the large proportion of girls in our study population, and the fact that our subjects were from two private schools (which may skew towards a higher socioeconomic grouping), our study still provides evidence that there may be an existing or emerging problem of iodine deficiency in school-age children in Australia. A larger cross-sectional study of schoolchildren is required to confirm the prevalence and severity of iodine deficiency in this population.
Analysis of urinary iodine values showed significantly more girls had iodine deficiency. This may be due to the unequal proportions of girls and boys in our study, and it would be important to determine whether this difference is reproduced in a larger study population. There is no evidence in the literature that girls are more susceptible to iodine deficiency. In the National Health and Nutrition Examination surveys in the United States, mean urinary iodine values were lower in girls than boys of a similar age group.28 There may be an unidentified effect of the female sex or sample bias on iodine homoeostasis. Perhaps boys are more likely to have a diet rich in dairy products. In a Dutch study, sex differences in urinary iodine excretion were attributed to boys consuming more iodine-supplemented bread and milk.29 In our study, further statistical analysis adjusting for sex and school year also showed a significant age-dependent effect on urinary iodine values.
The lack of relationship between goitre size and urinary iodine levels may be a consequence of our small cohort size, or it may confirm previous studies showing that the use of thyroid palpation as an isolated measure to assess iodine deficiency results in the mild iodine deficiency group remaining undetected.26,30 We would recommend the use of ultrasonography in a larger population study.
The minimum recommended daily iodine intake varies with age, but ranges from 120 μg in school-age children to 150 μg in adults. A urinary iodine excretion of 100 μg/L corresponds to a daily intake of 150 μg of iodine.25 The accumulating evidence for iodine deficiency in cohorts of our population suggests that iodine intake may be much lower than this.
A number of reasons for this change in iodine intake have been postulated. First, less salt has been consumed by the past two generations as a result of large-scale public health efforts to reduce hypertension and cardiovascular disorders in ageing populations. Second, iodised salt is not frequently used at home or in commercial food outlets. The range of preparations available in most food outlets suggests about a 4:1 availability of non-iodised versus iodised preparations. While the cost is similar, population awareness of a need for iodine supplementation is low. Finally, iodophors are no longer used in commercial milk-drum cleaning, which means that the iodine content of milk is now lower.
The presence of iodine deficiency in a population has significant implications. Mild iodine deficiency is associated with neurological deficits and hearing impairments.14-18,30 Studies from Sydney have indicated the emergence of iodine deficiency in large samples of neonates.1 Changing patterns of thyroid-stimulating hormone concentrations in newborns may provide another biological indicator of iodine deficiency in at-risk populations. Iodine deficiency in populations of schoolchildren and neonates raises the question of whether this reflects an increasing prevalence of iodine deficiency in our community as a whole. Larger, controlled studies of population subgroups would be able to answer this question.
If the presence of iodine deficiency is confirmed through a nationwide study, then a national program of iodine supplementation will need to be considered. The choice of vehicle is important because of health-related limitations on salt intake. Treatment of population iodine-deficiency states is ineffective unless the food supplemented with iodine can be distributed throughout the population.31 Whether bread or salt is chosen as the appropriate vehicle for iodine,32,33 distribution and social marketing remain the key to successful eradication of iodine deficiency.34,35
Our study has indicated the presence of mild iodine deficiency in a cohort of Melbourne schoolchildren. Our results support other available data on iodine status in Sydney and Tasmania to make a strong argument for a national study of iodine nutrition.
1: Iodine deficiency
Severe iodine deficiency results in goitre,5-9 and cretinism, with intellectual deficit, deaf mutism and severe physical disability (including the characteristic spasticity and rigidity),10,11 increased perinatal infant mortality and neonatal hypothyroidism,12 as well as decreased maternal fertility.13
Mild to moderate iodine deficiency can cause neurological deficits with learning disability and reduced hearing acuity in children14-18 and increased risk to premature infants, with reduced maternal transfer of iodine and thyroid hormone, which may result in impaired neural maturation.19,20
Iodine deficiency is the single most important cause of preventable intellectual deficit in the world.21
Maintained adequacy of iodine intake in populations is dependent on consuming food with sufficient natural iodine content or food supplemented with iodine.
2: Percentage and median urinary iodine values by sex, goitre assessment and school year in a sample of Melbourne schoolchildren
Category (number of subjects) |
Urinary iodine excretion (μg/L) |
||||||||||
25th quantile |
Median |
75th quantile |
< 50 μg/L |
50–99 μg/L |
≥ 100 μg/L |
||||||
Sex |
|
|
|
|
|
|
|||||
Male (n = 167) |
59 |
82 |
107 |
28 (17%) |
85 (51%) |
54 (32%) |
|||||
Female (n = 410) |
46 |
64 |
92 |
128 (31%) |
198 (48%) |
84 (21%) |
|||||
Total (n = 577) |
48 |
70 |
98 |
156 (27%) |
283 (49%) |
138 (24%) |
|||||
Grade of goitre* |
|
|
|
|
|
|
|||||
0 (n = 465) |
48 |
72 |
100 |
130 (28%) |
218 (47%) |
117 (25%) |
|||||
1 (n = 97) |
50 |
68 |
95 |
24 (25%) |
52 (54%) |
21 (22%) |
|||||
2 (n = 15) |
54 |
62 |
79 |
2 (13%) |
13 (87%) |
0 (0) |
|||||
School year |
|
|
|
|
|
|
|||||
5 (n = 34) |
55 |
69 |
101 |
4 (12%) |
21 (62%) |
9 (26%) |
|||||
6 (n = 25) |
50 |
71 |
100 |
6 (24%) |
12 (48%) |
7 (28%) |
|||||
7 (n = 117) |
42 |
70 |
92 |
39 (33%) |
55 (47%) |
23 (20%) |
|||||
8 (n = 108) |
52 |
74 |
113 |
25 (23%) |
47 (44%) |
36 (33%) |
|||||
9 (n = 87) |
54 |
76 |
98 |
16 (18%) |
50 (58%) |
21 (24%) |
|||||
10 (n = 69) |
48 |
67 |
91 |
20 (29%) |
35 (51%) |
14 (20%) |
|||||
11 (n = 74) |
41 |
67 |
95 |
22 (30%) |
35 (47%) |
17 (23%) |
|||||
12 (n = 63) |
43 |
62 |
83 |
24 (38%) |
28 (44%) |
11 (18%) |
|||||
* Grade 0: no palpable or visible goitre. Grade 1: palpable but not visible goitre. Grade 2: palpable and visible goitre. |
|||||||||||
4: Median urinary iodine values in schoolchildren, by grade of goitre*
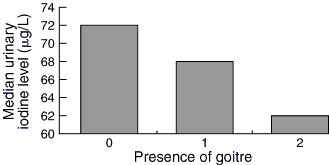
* Grade 0: no palpable or visible goitre.
Grade 1: Palpable but not visible goitre.
Grade 2: Palpable and visible goitre.
5: Logistic regression analysis comparing urinary iodine values < 50 μg/L with values > 50 μg/L, when adjusted for school year and sex
School year |
Odds ratio |
95% CI |
P |
||||||||
6 |
2.4 |
0.59–9.51 |
0.224 |
||||||||
7 |
5.5 |
1.78–17.10 |
0.003 |
||||||||
8 |
3.2 |
1.02–10.08 |
0.047 |
||||||||
9 |
2.2 |
0.68–7.24 |
0.188 |
||||||||
10 |
3.8 |
1.17–12.14 |
0.027 |
||||||||
11 |
3.6 |
1.13–11.46 |
0.030 |
||||||||
12 |
5.0 |
1.56–15.94 |
0.007 |
||||||||
Received 22 April 2002, accepted 17 October 2002
- Ciara M McDonnell1
- Mark Harris2
- Margaret R Zacharin3
- Department of Endocrinology and Diabetes, Royal Children's Hospital, Parkville, VIC.
We are indebted to Professor Cres Eastmann, Gary Ma and the staff of the ICPMR Laboratory, NSW, for performing the urinary iodine assays.
We also wish to thank the staff of the Clinical Epidemiology and Biostatistics Unit at the Royal Children's Hospital for their advice and assistance with the statistical analysis.
None identified.
- 1. McElduff A, McElduff P, Gunton JE, et al. Neonatal thyroid-stimulating hormone concentrations in Northern Sydney: further indications of mild iodine deficiency? Med J Aust 2002; 176: 317-320. <MJA full text>
- 2. Li M, Ma G, Guttikonda K, et al. Re-emergence of iodine deficiency in Australia. Asia Pacific J Clin Nutr 2001; 10: 200-203.
- 3. Gunton JE, Hams G, Fiegert M, McElduff A. Iodine deficiency in ambulatory participants at a Sydney teaching hospital: is Australia truly iodine replete? Med J Aust 1999; 171: 467-470. <MJA full text>
- 4. Guttikonda K, Burgess JR, Hynes K, et al. Recurrent iodine deficiency in Tasmania, Australia: a salutary lesson in sustainable iodine prophylaxis and its monitoring. J Clin Endocrinol Metab 2002; 87: 2809-2815.
- 5. Delange F. The disorders induced by iodine deficiency. Thyroid 1994; 4: 107-128.
- 6. Pharoah POD, Buttfield IH, Hetzel B. Neurological damage to the foetus resulting from severe iodine deficiency during pregnancy. Lancet 1971; 1: 308-310.
- 7. Thilly CH, Delange F, Lagasse R. Foetal hypothyroidism and maternal thyroid status in severe endemic goitre. J Clin Endocrinol Metab 1978; 47: 354-360.
- 8. Topliss DJ. Iodine deficiency disorders. Med J Aust 1989; 150: 669-671.
- 9. Chaouki ML, Maoui R, Benmiloud M. Comparative study of neurological and myxoedematous cretinism associated with severe iodine deficiency. Clin Endocrinol 1988; 28: 399-408.
- 10. Boyages SC. Clinical review 49: iodine deficiency disorders. J Clin Endocrinol Metab 1993; 77: 587-591.
- 11. Halpern JP, Boyages SC, Maberly GF, et al. The neurology of endemic cretinism. A study of two endemias. Brain 1991; 114: 825-841.
- 12. Delong GR, Stanbury JB, Fierro-Benitez R. Neurological signs in congenital iodine deficiency disorder (endemic cretinism). Dev Med Child Neurol 1985; 27: 317-324.
- 13. Dillon JC, Milliez J. Reproductive failure in women living in iodine deficient areas of West Africa. BJOG 2000; 107: 631-636.
- 14. Boyages SC, Collins JK, Maberly GF, et al. Iodine deficiency impairs intellectual and neuromotor development in apparently normal persons. A study of rural inhabitants of north-central China. Med J Aust 1989; 150: 676-682.
- 15. Fenzi GF, Giusti LF, Aghini-Lombardi F, et al. Neuropyschological assessment in schoolchildren from an area of moderate iodine deficiency. J Endocrinol Invest 1990; 13: 427-431.
- 16. Azizi F, Sarshar A, Nafarabadi M, et al. Impairment of neuromotor and cognitive development in iodine deficient schoolchildren with normal physical growth. Acta Endocrinol 1993; 129: 501-504.
- 17. Azizi F, Kalani H, Kimiagar M, et al. Physical, neuromotor and intellectual impairment in non-cretinous schoolchildren with iodine deficiency. Int J Vitamin Nutr Res 1995; 6199-205.
- 18. Tiwari BD, Godbole MM, Chattopadhyay N, et al. Learning disabilities and poor motivation to achieve due to prolonged iodine deficiency. Am J Clin Nutr 1996; 63: 782-786.
- 19. Ares S, Quero J, Dum S, et al. Iodine content of infant formulas and iodine intake of premature babies. Arch Dis Child 1994; 71: F184-191.
- 20. DeVries LS, Heckmatt J, Burrin JM, et al. Low serum thyroxine concentrations and neural maturation in preterm infants. Arch Dis Child 1986; 61: 862-866.
- 21. Hetzel BS. Iodine deficiency disorders and their eradication. Lancet 1983; 2: 1226-1229.
- 22. Eastman CJ. The status of iodine nutrition in Australia. In: Delange F, Dunn JT, Glinoer D. Iodine deficiency in Europe — a continuing concern. New York: Plenum Press, 1993: 133-139.
- 23. Eastman CJ. Where has all our iodine gone? Med J Aust 1999; 171: 455-456. <MJA full text>
- 24. Zacharin MR. Iodine deficiency and goitre in an urban population of children and adolescents, Victoria 2000. Proceedings of the Australasian Paediatric Endocrine Group; 2000 Oct 27-29; Sydney.
- 25. World Health Organization. Progress towards the elimination of iodine deficiency disorders (IDD). WHO/NUT/99.4 Geneva: WHO, 1999.
- 26. Vitti P, Martino E, Aghini-Lombardi F, et al. Thyroid volume measurement by ultrasound in children as a tool for the assessment of mild iodine deficiency. J Clin Endocrinol Metab 1994; 79: 600-603.
- 27. World Health Organization and International Council for Control of Iodine Deficiency Disorders. Recommended normative values for thyroid volume in children aged 6-15 years. Bull World Health Organ 1997; 75: 95-97.
- 28. Hollowell JG, Staehling NW, Hannon WH, et al. Iodine nutrition in the United States. Trends and public health implications: iodine excretion data from National Health and Nutrition Examination Surveys I and III (1971-74 and 1988-94). J Clin Endocrinol Metab 1998; 83: 3401-3408.
- 29. Wiersinga WM, Podoba J, Srbecky M, et al. A survey of iodine intake and thyroid volume in Dutch schoolchildren: reference values in an iodine sufficient area and the effect of puberty. Eur J Endocrinol 2001; 144: 595-603.
- 30. Knudsen N, Bulow I, Jorgensen T, et al. Goitre prevalence and thyroid abnormalities at ultrasonography: a comparative epidemiological study in two regions with slightly different iodine status. Clin Endocrinol 2000; 53: 479-485.
- 31. Lamberg BA. Endemic goitre-iodine deficiency disorders. Ann Med 1991; 23: 367-372.
- 32. Hintze G, Emrich D, Richter K, et al. Effect of voluntary intake of iodinated salt on prevalence of goitre in children. Acta Endocrinol 1988; 117: 333-338.
- 33. Gibson HB. Surveillance of iodine deficiency disorders in Tasmania 1949-1984. Hobart: Department of Health Services.
- 34. Delange F, Van Onderbergen A, Shabana W, et al. Silent iodine prophylaxis in Western Europe only partly corrects iodine deficiency; the case of Belgium. Eur J Endocrinol 2000; 143: 189-196.
- 35. Maberly GF. Iodine deficiency disorders: contemporary scientific issues. J Nutr 1994; 124 (Suppl): S1473-S1478.





Abstract
Objective: To assess iodine status and goitre prevalence in a sample of schoolchildren in Melbourne.
Design: Cross-sectional study of urinary iodine excretion and presence of goitre in a sample of schoolchildren from Years 5–12 attending two urban schools.
Participants: 607 children aged 11–18 years consented to thyroid gland palpation and 577 provided a urine sample on the day of examination in August 2001.
Outcome measure: Iodine status of the study population, based on median urinary iodine values categorised as normal (≥ 100 μg/L), mild (50–99 μg/L) or moderate–severe (< 50 μg/L), and classified according to sex, school year and presence of goitre.
Results: 76% (439/577) of students had abnormal urinary iodine values, with 27% (156/577) having values consistent with moderate–severe deficiency. The median urinary iodine excretion for the total group was 70μg/L, with values for school years 5–12 ranging from 62 μg/L (Year 12) to 76 μg/L (Year 9). The median urinary iodine value in girls was lower than that in boys (64μg/L v 82 μg/L), and girls had significantly lower urinary iodine values overall (P < 0.002). There was no association between goitre grade and moderate–severe (< 50 μg/L; P = 0.39) or mild (50–99 μg/L; P = 0.07) urinary iodine deficiency.
Conclusions: We found mild iodine deficiency in a cohort of schoolchildren in Melbourne. Our results support other data showing mild iodine deficiency in Sydney and Tasmania and the argument for a national study of iodine nutrition.