The conducting airways are lined by epithelium and their walls contain mucus-producing glands, cartilage, smooth muscle and connective tissue. A pseudostratified columnar epithelium, composed primarily of goblet and ciliated cells, lines the central airways and gradually reduces in height to form a low cuboidal lining in the distal conducting airways. A thin, viscous layer of fluid covering the epithelial surface of the conducting airways lies on top of a thicker layer of less viscous periciliary fluid. The bronchial mucus-secreting glands account for about 12% of the wall thickness in the mainstem bronchi and gradually diminish in both number and size in the peripheral conducting airways. They empty their secretions via ducts onto the airway surface. Cartilage makes up about 30% of the wall thickness in the mainstem and lobar bronchi and decreases to the extent where it is no longer present in the bronchioles. The bronchioles are defined, therefore, by the absence of either cartilage or mucous glands, and represent the smallest purely conducting airways in the tracheobronchial tree. Smooth muscle makes up about 5% of the airway-wall thickness in the mainstem bronchus, increasing gradually to about 20% of the wall thickness in the bronchioles. Respiratory bronchioles are defined by the presence of alveolar openings on their luminal surface. The number of these openings increases progressively down the bronchial tree until they cover the entire airway surface in the alveolar ducts and sacs.
The bronchial blood supply comes from the aorta and intercostal arteries, which feed a plexus of relatively large vessels on the outer wall of the airways. This plexus is connected by vessels passing through the muscle to a plexus of smaller vessels in the submucosa (see Box). These adventitial and submucosal vascular plexuses are perfused in series. Most of the arterioles that supply the submucosal vessels and the veins that drain it are in the adventitia and most of the capillary-sized vessels are in the submucosa. Blood supplying the central airways drains via bronchial veins into the azygous and hemiazygous systemic venous system, whereas the blood supply to the peripheral airways drains into the pulmonary veins. This vascular system conveniently divides the airway wall into adventitial and submucosal compartments that are supplied by separate sets of vessels and could mount different types of inflammatory reaction.1 This convenient compartmentalisation has been used by those investigating airway pathology, who refer to the "inner airway wall" (submucosa) and the "outer airway wall" (adventitia).2
It is now generally accepted that alterations in airway-wall smooth muscle and marked remodelling of the airway wall play an important role in the pathophysiology of asthma in adults. Studies of asthma pathology suggest that the remodelling process thickens both the inner and outer airway wall. Thicker airway walls are thought to intrude upon the airway lumen, causing narrowing and airflow limitation. Remodelling of the airway wall may also change its mechanical properties, thus contributing to altered luminal calibre. Indeed, postmortem studies have shown that, in the airways of people with asthma, wall thickening alone can explain a substantial proportion of the airway hyperresponsiveness that is characteristic of asthma.3 Examples of airway-wall remodelling that have been well documented in adults with asthma include increase in smooth muscle area and in the number and size of blood vessels in the airway wall, goblet-cell hyperplasia, airway-wall oedema, epithelial-cell disruption, and thickening of the subepithelial basement membrane collagen layer.
It has been clear for some time that asthma is an inflammatory disease. Studies have consistently shown that the airway walls of people with asthma exhibit a predominantly eosinophilic inflammatory infiltrate, coupled with some tissue-bound mast cells, lymphocytes, and neutrophils. This inflammatory process adds an exudate of fluid and cells to the fluid lining the surface of the airways. The exudate is derived from the submucosal microvessels under the control of a large number of inflammatory mediators. Mucus is added from the epithelial glands and mucus-secreting cells in the surface epithelium. An accumulation of this mucus-containing inflammatory exudate in the lumen is commonly referred to as "mucus plugging" of the airway, a characteristic feature of asthma that may be a key component of fatal asthma episodes. In a study by Kuyper et al,4 people with asthma were found to have a mean 37% (SD, 18%) occlusion of asthmatic airways by mucus and 23% (SD, 22%) occlusion by cells, compared with 6% (SD, 15%) and 3% (SD, 3%), respectively, in people without asthma.
The few studies of asthma pathology in children have not allowed the precise nature or magnitude of airway-wall remodelling in childhood to be defined. In one study carried out 25 years ago, endobronchial biopsies from two children with asthma showed all the hallmark pathological signs of asthma (ie, mucus plugging, goblet-cell hyperplasia, thickening of the bronchial basement membrane, smooth muscle hypertrophy and an eosinophilic infiltrate).5 In another study, there was postmortem evidence of aberrant glandular function in the airways of a two-year-old child with asthma, but no evidence of an increase in smooth muscle content within the airway wall.6 Hyperplastic goblet cells have also been seen in biopsies from two of 10 children with asthma (mean age, 9.3 years), although the most obvious pathological feature seen in this study was a thickening of the airway basement membrane, with increased subepithelial collagen deposition.7 The superficial nature of an endobronchial biopsy specimen does not allow conclusions to be drawn about pathological changes in the entire airway wall. It is still unclear as to when the pathological changes characteristic of asthma first appear, but radiological assessments of children with asthma have shown that bronchial-wall thickening is present at an early age.8
There have been few, if any, systematic histological studies of inflammation in the airway wall of children with asthma. In the biopsy study referred to above,7 bronchial inflammation was present in 60% of the children, but the inflammation was more lymphocytic in nature than the eosinophilic inflammation seen in adults with asthma. The absence of a significant eosinophilic inflammation was also observed in biopsies from 23 children with asthma (aged 6–17 years) when compared with six biopsies from children without asthma, although in that study the children with asthma were being treated with both oral and inhaled corticosteroids.9 It is interesting to note that recent preliminary results have indicated that the composition of the inflammatory exudate found within the airway lumen of children who have died from an episode of asthma is not different from that seen in adults.4 How early in childhood airway inflammation develops is yet to be elucidated, and the conclusions from histological studies are likely to be limited by a lack of comparative "normal" airway tissue from children in this age group.
The small conducting airways are surrounded by alveoli, and it is assumed that the dimensions of the alveoli and the airways they surround change in unison. Studies of the growing lung have shown that the branching system of the conducting airways is complete at birth, but that alveolarisation continues after birth and is not complete until about the second year of life.10 This implies that the resting calibre of the airways is dynamic in the first two years of life while alveolarisation is occurring. A change in radius of a tube results in a change in flow through the tube that is proportional to the fourth power of the change in radius (ie, a change in radius of 2 units results in a change in flow of 16 units). Therefore, changes in airway calibre that accompany growth of the lung during the first two years of life have a significant effect on airflow, as shown by a very sharp increase in peripheral airway conductance at about two years of age.11 Thus, thickening of the airway wall and/or luminal occlusion in a child with asthma before the age of two years may dramatically modulate these growth-related changes in airflow, resulting in a significantly lower airflow during early childhood that is likely to become less significant as the linear dimensions of the airways increase with lung growth. Interestingly, maximal airflow rates, and hence airway calibre, are lower in non-asthmatic male prepubertal children than in female children of the same age and height.12 This suggests that any alteration in airway-wall structure, in particular a thickening of the wall in children with asthma, may have a more marked adverse effect on airflow in male children than in female children.
Vascular arrangement in the airway wall of an adult with asthma
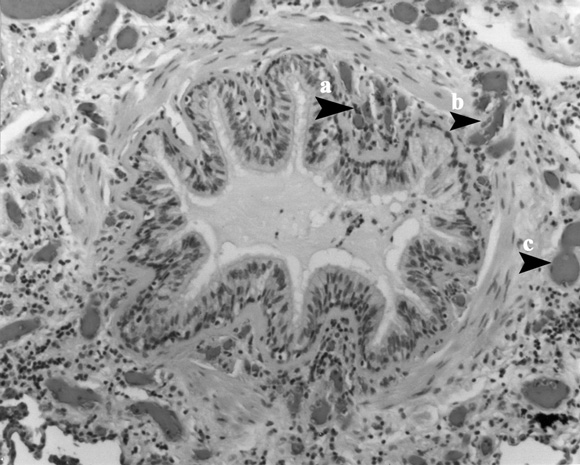
The vessels in the submucosa (a) are smaller than the vessels in the adventitia (c), and the vessels that pass through the muscle layer (b) connect them. These two sets of vessels are perfused in series, providing a basis for a difference in the nature of the inflammatory reaction in the submucosa and lumen compared with the peribronchiolar space.
- Karen O McKay1
- James C Hogg2
- Department of Respiratory Medicine and School of Paediatrics and Child Health, The Children's Hospital at Westmead, and University of Sydney, Westmead, NSW.
- 1 McDonald Research Laboratories, St Paul's Hospital, Vancouver, BC, Canada.
- 1. Kuwano K, Bosken CH, Paré PD, et al. Small airways dimensions in asthma and chronic obstructive lung disease. Am Rev Respir Dis 1993; 148: 1220-1225.
- 2. Bai A, Eidelman DH, Hogg JC, et al. Proposed nomenclature for quantifying subdivisions of the bronchial wall. J Appl Physiol 1994; 77: 1011-1014.
- 3. James AL, Paré PD, Hogg JC. The mechanics of airway narrowing in asthma. Am Rev Respir Dis 1989; 139: 242-246.
- 4. Kuyper IM, Paré PD, Hogg JC, et al. Characterization of the lumenal contents in fatal asthma [abstract]. Am J Respir Crit Care Med 2001; 163: A757.
- 5. Cutz E, Levison H, Cooper DM. Ultrastructure of airways in children with asthma. Histopathology 1978; 2: 417-421.
- 6. Engel S. Lung structure. Springfield, IL: Charles C Thomas, 1962: 53-55.
- 7. Cokugras H, Akcakaya N, Seckin I, et al. Ultrastructural examination of bronchial biopsy specimens from children with moderate asthma. Thorax 2001; 56: 25-29.
- 8. Hodson CJ, Trickey SE. Bronchial wall thickening in asthma. Clin Radiol 1960; 11: 183-191.
- 9. Payne DNR, Adcock IM, Wilson NM, et al. Relationship between exhaled nitric oxide and mucosal eosinophilic inflammation in children with difficult asthma, after treatment with oral prednisolone. Am J Respir Crit Care Med 2001; 164: 1376-1381.
- 10. Thurlbeck WM. Postnatal human lung growth. Thorax 1982; 37: 564-571.
- 11. Hogg JC, Williams J, Richardson JB, et al. Age as a factor in the distribution of lower-airway conductance and in the pathologic anatomy of obstructive lung disease. N Engl J Med 1970; 282: 1283-1287.
- 12. Hibbert M, Couriel JM, Landau LI. Changes in lung, airway and chest wall function in boys and girls between 8 and 12 years. J Appl Physiol 1984; 57: 304-308.





Abstract
What we knowWhat we need to know