|
Lessons from Practice
Childhood hepatotoxicity with paracetamol doses less than 150 mg/kg per day
MJA 1999; 171: 497
|
Paracetamol is widely used as an antipyretic and analgesic. Adverse
effects are regarded as unlikely at doses below 150 mg/kg per
day.1
However, lower doses have resulted in hepatotoxicity,2 and there is
growing evidence of the potential for hepatotoxicity in children
given multiple therapeutic or supratherapeutic doses of
paracetamol.3-6 The nomogram devised by Rumack and Matthews7 was based on data obtained
from previously well adult patients who had taken a single large dose
of paracetamol. The relevance of this to children given multiple
doses in the context of a febrile illness is unknown, particularly as
the metabolism in this population appears to be quite
different.8
It has been suggested that the therapeutic index for paracetamol may
be as low as 1.7,9 and that sick children under
the age of two years given in excess of 90 mg/kg per day for more than one
day should be regarded as being at higher risk.6 The product information
recommends a maximum daily dose of 60 mg/kg, but it is not uncommon for
children to receive doses in excess of 90 mg/kg per day in the hospital
setting.10
Although the number of reported cases of hepatotoxicity induced by
therapeutic doses of paracetamol is small, it is possible that cases
have gone unrecognised. It is important to administer the drug with
caution and according to current dosage guidelines.
| | | Case reports
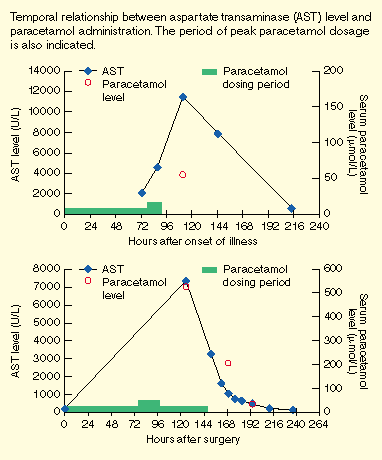
| | Case 1: Six days before transfer to our hospital, a previously well four-year-old, 20 kg girl had commenced a course of cefaclor for otitis media, and over 72 hours she received about 2400 mg of paracetamol in divided doses. She was admitted to her local hospital with fever (39ºC), abdominal pain, vomiting and diarrhoea. Her aspartate transaminase (AST) level was 2050 U/L (normal range, < 45 U/L). She was tachypnoeic and hypoxic, and over the next 17 hours received 2800 mg (140 mg/kg) paracetamol. Her condition deteriorated. Results of liver function tests were: AST, 4580 U/L (Figure A); alanine transaminase, 2785 U/L (normal range, < 55 U/L); and serum bilirubin, 27 µmol/L (normal range, < 15 µmol/L). The international normalised ratio of prothrombin time was 4.2, and activated partial thromboplastin time, 47 s (control, < 42 s). Left lower lobe pneumonia was diagnosed, and treatment commenced with fresh frozen plasma, vitamin K, and antibiotics. The AST level rose to 11 475 U/L. The paracetamol level 22 hours after the last documented dose of the drug was 55 µmol/L. N-acetylcysteine (150 mg/kg) was administered intravenously.
After the child was transferred to our hospital, intravenous N-acetylcysteine was continued (10 mg/kg/h for 32 h). Abdominal ultrasound revealed a large homogeneous liver and a small amount of ascites. Serology for hepatitis A and B, Epstein-Barr virus, cytomegalovirus and Mycoplasma pneumoniae was negative. Respiratory syncytial virus was detected in a nasopharyngeal aspirate. Blood cultures were negative. Stool examination revealed no viral agent.
The patient was discharged after seven days, with an AST level of 171 U/L. Three months later she was completely well, with normal liver function tests.
Case 2: A 12-year-old, 43 kg boy with Duchenne's muscular dystrophy was admitted for posterior spinal fusion and tendon-release surgery. He was anaesthetised using propofol and nitrous oxide, and during the operation required transfusion for a one-litre blood loss. He returned to the ward on a morphine infusion (20 µg/kg/h) and cephazolin (1 g eight-hourly). Over the next 24 hours he received a total dose of 3000 mg (70 mg/kg) paracetamol rectally. Similar total doses were given over the next five days, with a maximum of 4650 mg (108 mg/kg) in any 24-hour period. Liver function tests taken the day after surgery revealed an AST level of 193 U/L (Figure B) and a gamma-glutamyl transpeptidase (GGT) level of 43 U/L (normal range, < 40 U/L). He developed paralytic ileus 48 hours after surgery; this resolved with intravenous hydration.
On day seven, he became irritable and disoriented and was pale, icteric and lethargic. Results of investigations were: serum bilirubin, 120 µmol/L; GGT, 68 U/L; AST, 7377 U/L; and ammonia, 88 µmol/L (normal range, < 50 µmol/L). His serum paracetamol level was 528 µmol/L. The haemoglobin level was 68 g/L and he received two units of packed cells. Serology for hepatitis B and C was negative. The paracetamol level was 206 µmol/L 34 hours after the last dose, but, as the liver enzyme levels were falling and the child's conscious state improving, N-acetylcysteine was not administered. He was discharged 22 days after surgery with an AST level of 113 U/L.
|
| |  | | Back to text | |
Jenny L Hynson,* Mike South**
* Consultant Paediatrician
** Associate Professor, and Director
Department of General Paediatrics, Royal Children's Hospital
Flemington Road, Parkville, VIC 3052
- Rumack BH. Acetaminophen overdose in young children. Am J Dis
Child 1984; 138: 428-433.
-
Schoidt FV, Rochling FA, Casey DL, Lee WM. Acetaminophen toxicity
in an urban county hospital. N Engl J Med 1997; 337: 1112-1117.
-
Heubi JE, Barbacci MB, Zimmerman HJ. Therapeutic misadventures
with acetaminophen: hepatotoxicity after multiple doses in
children. J Pediatr 1998; 132: 22-27.
-
Alonso EM, Sokol RJ, Hart J, et al. Fulminant hepatitis associated
with centrilobular hepatic necrosis in young children. J
Pediatr 1995; 127: 888-894.
-
Rivera-Penera T, Gugig R, Davis J, et al. Outcome of acetaminophen
overdose in pediatric patients and factors contributing to
hepatotoxicity. J Pediatr 1997; 130: 300-304.
-
Kearns GL, Leeder JS, Wasserman GS. Acetaminophen overdose with
therapeutic intent [editorial]. J Pediatr 1998; 132: 5-8.
-
Rumack BH, Matthews H. Acetaminophen poisoning and toxicity.
Pediatrics 1975; 55: 871-876.
-
Penna A, Buchanan N. Paracetamol poisoning in children and
hepatotoxicity. Br J Clin Pharmacol 1991; 32: 143-149.
-
Heubi JE, Bien JP. Acetaminophen use in children: more is not better
[editorial]. J Pediatr 1997; 130: 175-177.
-
Penna AC, Dawson KP, Penna CM. Is prescribing paracetamol "pro re
nata" acceptable? J Paediatr Child Health 1993; 29: 104-106.
|
|





