HIV disease progression in Australia in the time of combination antiretroviral therapies
Patricia K Correll, Matthew G Law, Ann M McDonald, David A Cooper and John M Kaldor
MJA 1998; 169: 469-472
For editorial comment, see Stewart
Abstract -
Introduction -
Methods -
Results -
Discussion -
Acknowledgements -
References -
Authors' details
-
-
More articles on Infectious diseases and parasitology -
©MJA1998
Abstract |
Objective: To examine the effect of recent
developments in antiretroviral therapy on HIV disease progression
and survival. Design: Retrospective cohort study. Participants and setting: Two cohorts of people with HIV were defined retrospectively from the records of a large immunology laboratory. The first cohort were subjects whose CD4+ T cell counts had dropped to 200 x 106/L during 1990, and the second were subjects whose CD4+ T cell counts had dropped to 200 x 106/L in 1994. Main outcome measures: HIV disease progression and survival was determined over a minimum three years of follow-up for each cohort (ie, 1990-1993; 1994-1997). Results: 346 subjects were included in the analysis (193 subjects from 1990 and 153 from 1994). The relative risk of progression to AIDS in the 1994 cohort compared with the 1990 cohort was 0.57 (95% confidence interval, 0.35-0.91; P = 0.018) and the relative risk of death was 0.20 (95% confidence interval, 0.08-0.49; P < 0.001). Conclusions: There were 43% fewer AIDS cases and 80% fewer deaths in the time following the increased availability of combination antiretroviral therapy in Australia. |
Introduction |
In recent years there have been major advances in antiretroviral
treatments for people with HIV infection. Evidence from clinical
trials (DELTA and ACTG 175)1,2 first defined the
superiority of combination therapy over monotherapy in delaying
progression to AIDS and prolonging survival. Since that time more
potent antiretroviral agents have been approved, including
lamivudine (widely available in Australia since early 1995) and
protease inhibitors (available since late 1995). In clinical
trials, these drugs have demonstrated significant ability to reduce
disease progression and improve survival when added to existing
regimens of antiretroviral treatments.3,4 Although there has been anecdotal evidence in Australia suggesting that these treatments have reduced the overall number of AIDS cases and improved survival following AIDS, their effect has not been systematically studied. We therefore decided to compare the rates of progression to AIDS and survival in two cohorts of people between 1990-1993 and 1994-1997, with CD4+ T cell counts of 200 x 106/L (CD4 cell count 200/µL) at entry to the study, as a basis for assessing the impact of new treatments. |
Methods |
Subject identificationTwo cohorts of subjects in this study with CD4 cell counts of 200/µL were defined retrospectively from records of routine CD4 cell counts undertaken at the Centre for Immunology, St Vincent's Hospital, Sydney. St Vincent's Hospital has been responsible for the care of about 25% of people with AIDS in Australia, and its immunology laboratory is used by St Vincent's Hospital inpatient and outpatient services, and by several general practitioner clinics that specialise in managing people with HIV infection.Subjects whose CD4 cell counts fell below 200/µL in 1990 would not have had access to combination therapies, whereas the 1994 cohort was under follow-up when combination therapies with more potent drugs were becoming available in Australia. A CD4 cell count of 200/µL was used as the starting point for follow-up because at this point individuals have not usually developed AIDS-defining illnesses,5 but are considered to be at a point of immune decline where the probability of developing AIDS in the near future has greatly increased.6,7 Subjects included were HIV-positive but without AIDS at entry to the study, with CD4 cell counts declining through 200/µL in the index year (1990 or 1994) or with a single CD4 cell count in the range 180-220/µL. All eligible subjects were included, except subjects from two large clinics which had used the Centre for Immunology laboratory in 1990 but not in 1994. Where available, two or more CD4 cell count results from the period beginning in the middle of the year before the index year and ending at the middle of the following year were recorded so that the rate of CD4 cell change could be estimated. Subjects with multiple CD4 cell counts entered the study on the date of the first test in the index year that was below 200/µL. For subjects with a single CD4 cell count only, entry to the study was on the date of this test, provided the result fell between 180-220/µL.
Follow-upAnalysis was based on a minimum of three (up to four) years' follow-up of both cohorts through linkage to the National AIDS Registry, which records AIDS diagnoses and deaths following AIDS in Australia. Testing records were linked to Registry data by matching name codes (first two letters of the surname and given name) and dates of birth from the reference laboratory database with the Registry. Because reporting of AIDS diagnoses and deaths to the Registry is subject to delay, subjects from the 1994 cohort who did not appear on the Registry were followed up through their referring doctors. The doctor was also contacted for follow-up information if a name code was not available with the CD4 count records. Information sought included name code, date of birth, whether the subject had progressed to AIDS or died with AIDS, and date of the doctor's most recent contact with the subject.
Statistical methodsAll statistical analyses were performed using SAS.8 Decline of CD4 cells was estimated for subjects with two or more CD4 cell counts by linear regression. Baseline characteristics in the two cohorts were compared by means of Fisher's exact test for categorical variables and the Mann-Whitney rank sum test for continuous variables.We calculated AIDS-free survival and overall survival curves using Kaplan- Meier methods, and assessed the statistical significance of the difference in survival between the two cohorts by the log rank test. Proportional hazards regression was used to assess the effect of age, CD4 cell count at entry, and decline of CD4 counts on AIDS incidence and survival. In survival analysis, subjects without an AIDS diagnosis or death reported to the National AIDS Registry were assumed to be AIDS-free and alive at the end of follow-up. Secondary analyses were also performed based on confirmed AIDS-free and survival times by censoring at the date of last confirmed contact on the National AIDS Registry or with the referring doctor. |
Results |
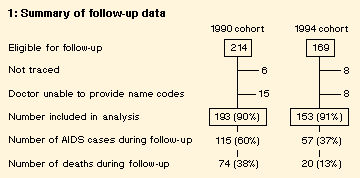
After excluding 109 subjects in 1990 who had been referred from two
clinics which did not use the Centre for Immunology laboratory in
1994, the database search identified 214 eligible subjects from 1990
and 169 from 1994, of whom 193 and 153, respectively, could be traced
for follow-up and were included in the analysis (Box 1).
As shown in Box 1, 60% of subjects who were followed up during 1990-1993 progressed to AIDS and 38% died, compared with 37% who progressed to AIDS and 13% who died during follow-up between 1994-1997. This corresponds to a 43% decrease in AIDS and an 80% reduction in death.
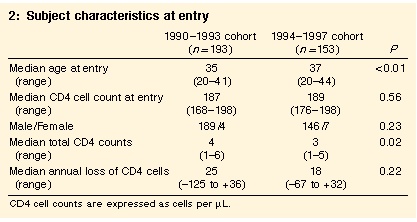
As shown in Box 2, the median age at entry of the 1994 cohort was older than in the 1990 cohort, but sex, CD4 cell count at entry and CD4 cell decline were similar. The median age and sex of excluded subjects who came from the two clinics which did not use the Centre for Immunology laboratory in 1994 (age 37.5 years, 96% male) were similar to those included in the 1990 cohort (age 35 years, 98% male).
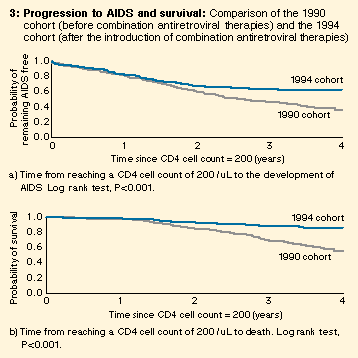
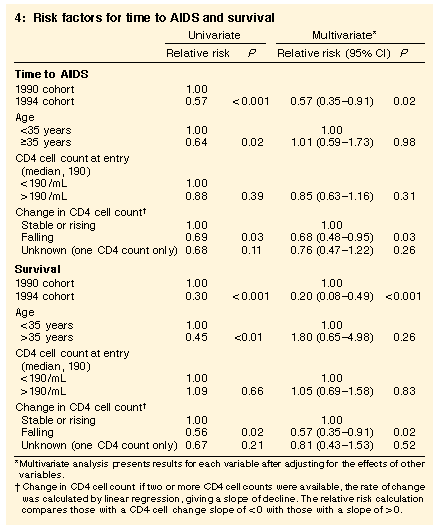
Box 3 shows that progression to AIDS was slower and survival longer in the 1994 cohort than in the 1990 cohort, and that the improvement in both indices appeared to emerge after about one year of follow-up. Adjustment for age, CD4 count and CD4 slope at entry did not materially affect the differences between the two cohorts in progression time to AIDS and survival (Box 4).
There were 52 subjects for whom only one CD4 cell count was recorded and rate of CD4 cell decline could not be estimated. A sensitivity analysis excluding these subjects gave very similar results to those for the full cohort (relative risk of AIDS and death in the 1994 group, 0.51 [P = 0.02] and 0.21 [P < 0.01], respectively). When follow-up was censored at the date of last confirmed contact, there remained a 28% decrease in progression to AIDS (P = 0.04) and a 45% reduction in death (P = 0.021). |
Discussion |
In this study, progression to AIDS was nearly halved and deaths fell by
80% among people with HIV infection whose CD4 cell count fell below
200/µL in 1994 compared with the corresponding 1990 cohort.
The difference between the two groups emerged after about one year of
follow-up, which in the 1994 cohort corresponded roughly to the
introduction of lamivudine in early 1995, followed by protease
inhibitors later in the same year. Although other differences
between the cohorts cannot be entirely ruled out, the most likely
explanation for the improved outcomes is advances in treatment
strategies for people with HIV, including combination
antiretroviral therapies.
The benefit of combination therapy and new antiretroviral agents has been demonstrated so far largely through randomised controlled trials.9 Only recently has information started to emerge of the effectiveness of new therapies in a community-wide setting. The results of this study concur with recent cohort studies overseas, where substantial decreases in AIDS and deaths have been identified in patients followed up after the introduction of combination therapies compared with those under follow-up in earlier times.10,11 Although information on antiretroviral treatment was unavailable for the individuals in our cohorts, recent evidence suggests a dramatic change in the use of antiretroviral therapies in Australia over the time in which the two cohorts reported in our study were followed up. HIV-infected participants in the Sydney Men and Sexual Health Study (a prospective study of homosexually active men in Sydney) increased their uptake of combination antiretroviral therapy from about 2% in 1993 to more than 70% in 1997.12 Our study relied on the National AIDS Registry, which is subject to both reporting delay and underreporting. It is believed that about 70% of HIV diagnoses are reported to the Registry within six months (the minimum reporting time available in this study) and that nearly 100% are reported within three years.13 It is possible that incomplete reporting of AIDS and AIDS-related deaths to the National AIDS Registry may have resulted in underestimates of AIDS incidence or mortality in the later cohort. Results were similar, however, when analyses were censored at the date of last contact, suggesting that underreporting to the Registry was not in fact an important source of bias. One other potential source of bias is that changes in the natural history of the HIV epidemic may have resulted in a lower proportion of rapid progressors in more recent years, so that patients with CD4 counts of 200/µL in 1990 progressed more rapidly than those in 1994.14 In our analysis, there was little difference between the cohorts in CD4 cell decline (Box 2), and survival analysis adjusted for this gave very similar results. Furthermore, a sensitivity analysis excluding those subjects with only a single CD4 cell count also did not significantly affect the results. This analysis was based on CD4 cell counts collected over two years (mid year before the index year to mid year after), and is robust enough to suggest that both cohorts were subject to similar rates of progression. As the subjects were identified at two different times, it is possible that there were unidentifiable differences between the cohorts that may have influenced the outcomes in this study. However, the possibility of bias was substantially reduced by identifying all subjects from the same laboratory. This study contributes evidence to suggest that the rate of progression of HIV disease has decreased in Australia at the same time as new, more potent combination antiretroviral treatments have become available. It would be useful to study similar cohorts in the future to monitor the evolving pattern of the epidemic at a population level. |
Acknowledgements | The National Centre in HIV Epidemiology and Clinical Research is funded by the Commonwealth Department of Health and Family Services through the Australian National Council on AIDS and Related Diseases. The help of the following medical practitioners in study follow-up is gratefully acknowledged: B Anderson, P Brooke, K Brown, A Carr, B Donovan, N Doong, C Duncombe, W Genn, J Kidd, A Mackie, M McMurchie, A McNulty, R Penny, A Pethebridge, M Robertson. We also thank John Zaunders at the St Vincent's Hospital Centre for Immunology for assistance in identifying the study sample. |
References |
(Received 13 Jan, accepted 30 Apr, 1998) |
Authors' details
National Centre in HIV Epidemiology and Clinical Research, Sydney, NSW.Patricia K Correll, BN, MPH, Research Assistant;
Matthew G Law, MA, MSc, Statistician;
Ann M McDonald, BSc, MPH, Senior Research Assistant;
David A Cooper, DSc, MD, FRACP, Director;
John M Kaldor, PhD, Deputy Director.
Reprints will not be available from the authors.
Correspondence: Patricia Correll, National Centre in HIV
Epidemiology and Clinical Research, Level 2, 376 Victoria Street,
Sydney, NSW 2010.
Email: pcorrellATnchecr.unsw.edu.au
-
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
Received 4 March 2026, accepted 4 March 2026
- Patricia K Correll
- Matthew G Law
- Ann M McDonald
- David A Cooper
- John M Kaldor