Synopsis
- With increasing implementation of casemix-based funding for
hospitals, quantitative data were needed to confirm the clinical
impression that treating Aboriginal (compared with
non-Aboriginal) inpatients consumes significantly more
resources.
- Utilisation data, collected over a three-month period in 10
hospitals, were used to determine a cost per inpatient episode, which
was grouped according to AN-DRG-3 to give a cost per AN-DRG for
Aboriginal and Torres Strait Islander (ATSI) patients and non-ATSI
patients.
- ATSI patients had consistently longer average length of stay and
significant variation in relative frequency of admissions,
compared with non-ATSI patients, with higher prevalences of
infectious diseases. Degenerative and neoplastic conditions were
more common in non-ATSI patients.
- There were significant differences in casemix-adjusted costs per
patient episode (ATSI, $1856; non-ATSI, $1558; P <
0.001).
- Our study has quantified differential resource consumption between two Australian populations, and highlights the need for recognition of some hospitals' atypical populations and special funding requirements.
Introduction
There is substantial evidence in the medical literature of poor health outcomes for Aboriginal and Torres Strait Islander (ATSI) people despite high hospital utilisation rates.1 Among the reforms designed to improve health outcomes, casemix classification (Australian national diagnosis-related groups, AN-DRGs) for hospital inpatients could, on the contrary, have deleterious effects if its limitations were not appreciated.
The principle underpinning casemix systems -- that clinically similar patients consuming similar resources can be grouped into a DRG which will have an equal spread of patients consuming more and less resources -- means that a hospital with an atypical population will be inappropriately funded. Health service providers who treat patients from remote Aboriginal communities believe that treating Aboriginal patients is considerably more expensive for a range of reasons (severity of disease at presentation, comorbidities, and social factors relating to culture, education and remote location), but there are few data quantifying their resource consumption during inpatient care.
With increasing implementation of casemix, quantitative data were urgently needed, so that hospitals caring for such populations would receive appropriate funding. The first study attempting to quantify differential resource consumption of Aboriginal and non-Aboriginal patients2 had considerable methodological problems, resulting in the data being of limited use.
In 1993 the Australian Casemix Clinical Committee recommended to the (then) Commonwealth Department of Human Services and Health that a multicentre study be conducted to quantify differences in resource consumption patterns between ATSI and non-ATSI inpatients in rural and remote settings.
Methods In view of the complexity of the project, a representative steering committee was established to define the scope and provide clinical oversight for the proposed research.After an analysis of Australia-wide hospital morbidity data, including utilisation rates by ATSI patients, a sampling framework was developed. Ten hospitals of more than 30 beds from Western Australia (Kalgoorlie), Northern Territory (Royal Darwin, Katherine and Alice Springs), South Australia (Port Augusta) and Queensland (Cairns, Mount Isa, Cunnamulla, St George and Innisfail) agreed to participate as study sites.
External consultants (Brewerton and Associates, Adelaide) were appointed to facilitate data collection and analysis within the guidelines established by the steering committee.
Collection and review of data, and consultation
Data were collected from each site over a three-month period. Six
sites commenced collection on 1 July 1995. The remaining four sites
began one month later. For each patient in the study, a range of
detailed utilisation data was obtained. Specific proformas were
developed to collect details on nursing time, medical time,
diagnostic services (pathology and imaging) and therapeutic
services (theatre, pharmaceuticals, allied health). Additional
information on diagnosis, procedures and morbidity was obtained
from the hospitals' information systems.
The utilisation data were used to determine a cost per inpatient episode. The costed patient data were grouped according to AN-DRG-3 to produce a cost per AN-DRG for the two populations.
Traditional costing studies, which use cost information extracted from the hospital's general ledger and allocated to DRG classes, would not have provided costing information to the required level. Therefore, we used national unit prices to complete the cost allocation process (Box 1). This also overcame the lack of sophistication of many of the hospitals' cost reporting, and avoided the need to make accrual adjustments to hospitals' general ledgers for the three-month period. The national unit prices were based on national and State labour force data, and recently completed national casemix costing and service weight studies and analyses undertaken to generate AN-DRG-3 cost weights.3 This approach also removed idiosyncratic local cost variations and enhanced the reliability of the results. Thus, for the purposes of our study, costs such as those for a unit of nursing time, and individual radiology and pathology tests, were the same for all hospitals.
Patients were classified according to AN-DRG-3. To ensure satisfactory coding standards, a random audit of medical records was undertaken in each hospital before the commencement of data collection.
Interim results were compiled and presented at a workshop in Alice Springs in April 1996. Attendees included health service providers from the study hospitals and State Health Departments, as well as representatives from consumer groups, such as the National Aboriginal Community Controlled Health Organisation (NAACHO) and the Office of Aboriginal and Torres Strait Islanders (OATSI). As a result of this meeting the data were further refined, allowing for more clinically accurate and culturally appropriate interpretation. A final report was presented to the Commonwealth Department of Health and Family Services in April 1997.4
Ethical approval
Participating hospitals were required to consider the ethical
implications of the research project, and, in particular, issues of
confidentiality. The hospital data and the study report were not to
include any information identifying individual patients or
communities. At the conclusion of the study, hospitals were provided
with their own data in addition to that of the total cohort. No hospital
had access to another hospital's data unless by private arrangement.
Statistical analysis
Collation of data was facilitated by a specially designed
application using dBase as the programming tool. SPSS (SPSS Inc,
Chicago, Illinois, USA) and standard spreadsheet packages were used
for the analyses, which were based on t tests, as comparisons
were between two populations with large sample sizes. For both
populations, only those AN-DRGs with a sample size exceeding 20
separations were analysed.
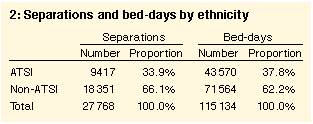
It was not possible to standardise the data by sex and age for the total study population, as population data for the hospitals' catchment areas were not available. Standardised data for the Northern Territory (not presented here) revealed higher admission rates for male and female ATSI patients compared with non-ATSI patients in all age groups.
While ATSI separations represented 33.9% of the total cohort they represented 66% (115/174) of patients and 67.6% (3157/4673) of separations of those assigned AN-DRG 572, Admit for renal dialysis. Because of the impact this caseload would have had on cost analysis (eg, one population would have a disproportionate number of "day-only" admissions), data relating to dialysis were excluded from relevant sections of the analysis. The average length of stay in the ATSI population was two days shorter when AN-DRG 572 was included in the analysis, but in the non-ATSI cohort it was only 0.3 days shorter.
Differences in DRGs
The ATSI population was distributed across 426 of a possible 667 (64%)
DRGs. In contrast, the non-ATSI population was distributed across
547 DRGs (82%). Box 3 highlights the consistently longer average
length of stay of ATSI patients, as well as a significant variation in
relative frequency of admissions. For example, DRGs for
gastroenteritis and respiratory infections contain more ATSI
patients, despite there being twice as many non-ATSI patients in the
cohort. In contrast, DRGs for gastroscopy and colonoscopy have a
higher proportion of non-ATSI patients. Dental extractions and
restorations recorded low separation rates in ATSI patients.
The following DRGs were not encountered in ATSI patients during the
data collection period: Other major joint and limb reattachment
procedures without comorbidities and complications; Major
shoulder or elbow procedures, age < 60; and Hip and femur
procedures except major joint, age > 54 without comorbidities and complications.
Boxes 4 and 5 show the top 20 DRGs by volume for ATSI and non-ATSI patients, respectively. Of note is the prevalence of infectious diseases in the ATSI population compared with the non-ATSI population, whereas the non-ATSI population has a high prevalence of degenerative diseases and DRGs related to neoplastic conditions.
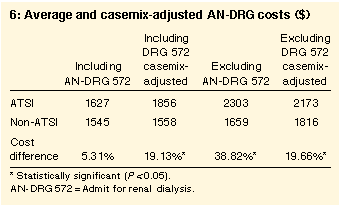
Cost differences
The unadjusted average cost of an ATSI inpatient episode was $1627
compared with $1545 for non-ATSI inpatient episodes (this
difference was not significant). The casemix-adjusted costs,
however, showed significant differences (P < 0.001) per
episode at $1856 and $1558 for ATSI and non-ATSI patients,
respectively (Box 6).
Box 7 shows the breakdown of total and average costs and confirms that the cost differential is a result of increased utilisation of most services. Theatre and pathology services are the only areas where costs are higher for non-ATSI patients. Further analysis of the data showed that operating room expenses were higher for ATSI patients. However, the average cost is lower because a significantly smaller number of ATSI patients had operations.
The data also confirm that ATSI patients have longer lengths of stay and higher costs in most Major Diagnostic Categories (MDCs) (Box 8). An unexpected observation was the shorter length of stay and cost for this population in MDC 19 (Mental Diseases and Disorders), and MDC 20 (Alcohol/Drug Use and Alcohol/Drug Induced Organic Mental Disorders).
Discussion The study confirmed the clinical perception that caring for ATSI inpatients consumed greater resources for the same DRG than caring for non-ATSI inpatients, and demonstrated a 39% overall differential cost. For some DRGs (eg, those including paediatric infectious diseases) the increase in resource consumption was considerable in ATSI patients. In MDCs 19 and 20, non-ATSI patients used slightly more resources.The greater costs in ATSI patients are believed to be related to disease severity on admission as well as comorbidities and complicating factors. Data obtained during the project support this.
Other studies have also found that resource utilisation for ATSI patients is lower for mental disorders.5 Easier reintegration of ATSI patients into their community may facilitate shorter lengths of stay. Social networks and supports may also favour outpatient psychiatric care. The same may be true for DRGs associated with alcohol abuse, although given the known prevalence and impact of substance abuse in ATSI patients, we may also be identifying a need for further review of the models of healthcare delivery to ATSI patients.
Actual needs were not addressed by our study. We only measured the current state of healthcare provision, which is largely a result of historical funding arrangements. However, many clinicians would argue that current health services for underprivileged groups are inadequate.
This is the first study to quantify differential resource consumption between two Australian populations. It highlights the need to recognise potentially confounding factors when a casemix classification funding system is implemented. The Northern Territory, South Australia and New South Wales have recognised the disparity and incorporated funding adjustments for ATSI patients.
As with ATSI patients in remote and rural hospitals, other socially disadvantaged groups including Aboriginals in urban settings and immigrant subpopulations may also have a cost and utilisation profile different from the "typical" Australian population. Hospitals caring for a significant proportion of such patients may equally need recognition for their "atypical" population. Appropriate funding of such hospitals can be either through funding adjustments or by an improved classification system.
Future versions of AN-DRGs are likely to make greater use of complicating clinical factors (CCFs), which could include indicators of social disadvantage. Notwithstanding these efforts, hospitals caring for atypical populations remain vulnerable because their relatively small number of patients lack statistical importance when national figures are reviewed.
One of the great challenges of casemix implementation is to provide the basis by which hospitals can be funded appropriately for appropriate care. If this challenge is not met it is the sickest patients from the most disadvantaged subpopulations who will suffer. The Aboriginal and Torres Strait Islander Casemix Study has demonstrated a genuine risk in this regard.
1: Standard unit of cost
| Unit cost per minute by nursing level | |
| Obtained from: | Market Basket Database: CDHS&H |
| Applied to: | Patient attributable time by nurse per patient |
| Unit cost per minute by allied health professional level | |
| Obtained from: | Market Basket Database: CDHS&H |
| Applied to: | Patient attributable time by allied health professional by patient |
| Unit cost by banded time range for medical officer | |
| Obtained from: | Banded ranges and standard cost as specified in the MBS schedule and adopted by the South Australian Health Commission |
| Applied to: | Frequency of consultations by time range by medical officer |
| Unit cost per operating minute by procedure | |
| Obtained from: | National Operating Room Service Weight Study: CDHS&H |
| Applied to: | Time spent in theatre and recovery rooms |
| Unit cost per day in intensive care/critical care/neonatal intensive care | |
| Obtained from: | National Intensive Care Service Weight Study: CDHS&H |
| Applied to: | Time spent in intensive care/critical care/neonatal intensive care |
| Unit cost per pathology test by type | |
| Obtained from: | National Pathology Service Weight Study: CDHS&H |
| Applied to: | Each pathology test ordered and undertaken per patient |
| Unit cost per diagnostic imaging service by type | |
| Obtained from: | National Diagnostic Imaging Service Weight Study: CDHS&H |
| Applied to: | Each diagnostic imaging procedure performed per patient |
| Unit cost per pharmaceutical by type | |
| Obtained from: | Average unit price based upon data provided from the participating sites |
| Applied to: | Drug type administered per patient by dosage and frequency |
| Unit cost per prosthesis by type | |
| Obtained from: | Standard List National Operating Room Service Weight Study: CDHS&H |
| Applied to: | Prostheses consumed in theatre |
| Unit overhead rate | |
| Obtained from: | Development of AN-DRG-3 Cost Weights: CDHS&H |
| Applied to: | Each day of stay, covering overhead costs plus each day of stay for a boarder |
| CDHS&H = Commonwealth Department of Human Services and Health (now, Commonwealth Department of Health and Family Services). MBS = Medical Benefits Schedule. |
8: Average cost by major diagnostic category (MDC) (including AN-DRG 572)
| MDC Description | ||||||
cost ($) | cost ($) | |||||
| 0 Pre MDC | ||||||
| 1 Diseases and Disorders of the Nervous System | ||||||
| 2 Diseases and Disorders of the Eye | ||||||
| 3 Diseases and Disorders of the Ear, Nose, Mouth and Throat | ||||||
| 4 Diseases and Disorders of the Respiratory System | ||||||
| 5 Diseases and Disorders of the Circulatory System | ||||||
| 6 Diseases and Disorders of the Digestive System | ||||||
| 7 Diseases and Disorders of the Hepatobiliary System and Pancreas | ||||||
| 8 Diseases and Disorders of the Musculoskeletal System and Connective Tissue | ||||||
| 9 Diseases and Disorders of the Skin, Subcutaneous Tissue and Breast | ||||||
| 10 Endocrine, Nutritional and Metabolic Diseases and Disorders | ||||||
| 11 Diseases and Disorders of the Kidney and Urinary Tract | ||||||
| 12 Diseases and Disorders of the Male Reproductive System | ||||||
| 13 Diseases and Disorders of the Female Reproductive System | ||||||
| 14 Pregnancy, Childbirth and the Puerperium | ||||||
| 15 Newborns and Other Neonates | ||||||
| 16 Diseases and Disorders of the Blood and Blood Forming Organs | ||||||
| 17 Myeloproliferative Diseases and Poorly Differentiated Neoplasms | ||||||
| 18 Infectious and Parasitic Diseases | ||||||
| 19 Mental Diseases and Disorders | ||||||
| 20 Alcohol/Drug Use and Alcohol/Drug Induced Organic Mental Disorders | ||||||
| 21 Injury, Poisoning and Toxic Effects of Drugs | ||||||
| 22 Burns | ||||||
| 23 Factors Influencing Health Status and Other Contacts with Health Service | ||||||
| Total | ||||||
| ATSI = Aboriginal and Torres Strait Islander. Seps = Separations. ALOS = Average length of stay (days). AN-DRG 572 = Admit for renal dialysis. |
3: Top 20 DRGs by volume - total study population
| AN-DRG and description | ||||||
| 572 Admit for renal dialysis | 4673 | 1.01 | 3157 | 1.02 | 1516 | 1.00 |
| 943 Other factors influencing health status | 1840 | 2.36 | 395 | 5.49 | 1441 | 1.49 |
| 727 Neonate, admission weight <2499g without significant operating room procedure, without problems | 994 | 3.70 | 268 | 4.25 | 726 | 3.50 |
| 674 Vaginal delivery without complicating diagnoses | 805 | 3.45 | 163 | 3.84 | 642 | 3.35 |
| 952 Ungroupable | 614 | 7.93 | 288 | 7.06 | 326 | 8.70 |
| 683 Abortion with D&C, aspiration curettage or hysterectomy | 515 | 1.15 | 70 | 1.46 | 445 | 1.10 |
| 332 Other gastroscopy for non-major digestive disease without comorbidities and complications | 380 | 1.41 | 25 | 2.32 | 355 | 1.34 |
| 686 Other antenatal admission with moderate or no complicating diagnoses | 357 | 2.44 | 88 | 3.57 | 269 | 2.07 |
| 172 Respiratory infections/inflammation, age <55 without comorbidities and complications | 351 | 4.78 | 245 | 5.24 | 106 | 3.72 |
| 350 Gastroenteritis age <10 | 338 | 5.77 | 188 | 8.88 | 150 | 1.85 |
| 187 Bronchitis and asthma age <50 without comorbidities and complications | 266 | 2.69 | 56 | 3.25 | 210 | 2.54 |
| 659 Conisation, vagina, cervix and vulva procedures | 259 | 1.57 | 35 | 2.69 | 224 | 1.39 |
| 780 Chemotherapy | 256 | 1.23 | 15 | 2.60 | 241 | 1.14 |
| 885 Injuries age <65 | 254 | 2.30 | 103 | 2.97 | 151 | 1.84 |
| 491 Cellulitis age <60 without comorbidities and complications | 222 | 4.14 | 91 | 5.37 | 131 | 3.29 |
| 484 Other skin, subcutaneous tissue and breast procedure | 213 | 1.98 | 32 | 4.50 | 181 | 1.53 |
| 335 Other colonoscopy without comorbidities and complications | 211 | 1.67 | 9 | 3.44 | 202 | 1.59 |
| 128 Dental extractions and restorations | 208 | 1.26 | 32 | 1.59 | 176 | 1.20 |
| 349 Oesophagitis/gastroenteritis/other digestive disease age 10-74 | 208 | 2.26 | 48 | 2.98 | 160 | 2.05 |
| 660 Endoscopic procedures, female reproductive system | 206 | 1.34 | 42 | 1.90 | 164 | 1.20 |
ATSI = Aboriginal and Torres Strait Islander. Seps = Separations. ALOS = Average length of stay (days)
- Plant AJ, Condon JR, Durling G. Northern Territory health outcomes, morbidity and mortality 1979-1991. Darwin: Northern Territory Department of Health and Community Services, 1995.
- Harkin K. Incremental resource consumption by Aboriginal inpatients: a research project conducted at Alice Springs Hospital from 1 October to 31 May,1992. Report to the Department of Human Services and Health. Darwin: NT Dept of Health and Community Services, 1994.
- Casemix Development Program. Report on the development of AN-DRG Version 3 Cost weights. Canberra: Commonwealth Department of Human Services and Health, 1995.
- Commonwealth Department of Health and Family Services. Report on National Aboriginal and Torres Strait Islander Casemix Study. Adelaide: Brewerton and Associates Pty Ltd, April 1997.
- Jablensky A. The epidemiology of schizophrenia. Curr Opin Psych 1993; 6: 43-52.
Dale A Fisher,* FRACP, DTM&H, Physician and Senior Lecturer.
Classification and Payments Branch, Department of Health and Family
Services, Canberra, ACT.
Jo M Murray,* BSc(Med), Acting Assistant Secretary.
Princess Alexandra Hospital, Brisbane, QLD.
Michael I Cleary,* FACEM, MHA, Executive Director of Medical
Services.
Brewerton and Associates, Adelaide, SA.
Rita E Brewerton, BSc(MaSc)Hons, Director.
Reprints will not be available from the authors.
Correspondence: Dr D
A Fisher, Royal Darwin Hospital, PO Box 41326, Casuarina, NT
0811.
E-mail: dale.fisherATnt.gov.au
*Steering Committee members (other members are listed in the Acknowledgements above).




