Prevalence of tuberculosis infection in Melbourne secondary school students
Paul D R Johnson, John B Carlin, Catherine M Bennett, Peter D Phelan
Michael Starr, Jane Hulls and Terry M Nolan
MJA 1998; 168: 106-110
Abstract -
Introduction -
Methods -
Statistical analysis -
Results -
Discussion -
Acknowledgements -
References -
Authors' details
-
-
©MJA1998
Abstract |
Objective: To estimate the prevalence of
asymptomatic Mycobacterium tuberculosis infection in
Melbourne secondary school students. Design: Cross-sectional Mantoux testing of a partly random and partly targeted sample of secondary school students, designed to enable estimation of prevalence by region of birth. Setting: Fifty-one State and Catholic secondary schools in metropolitan Melbourne during 1995. Participants: Australian and overseas-born students in Years 9 and 10. Outcome measures: Proportions of students with positive Mantoux reactions (defined as induration at 48 hours of ≥5 mm with a history of recent exposure; ≥10 mm and no prior BCG vaccination; ≥15 mm and prior BCG vaccination). Results: Of 2586 students potentially eligible for testing, evaluable results were obtained from 1274 (49%). The overall prevalence of infection for Melbourne students in Years 9 and 10 was 2.5% (95% CI, 1.1-3.9%). Main predictors of a positive test were birth overseas and number of years residing overseas. Prevalence varied considerably by region of birth, and was very low in students born in Australia (0.7%), "other developed countries" (0.7%), and Southern Europe (0). The highest rates were observed in students born in Indochina (15.9%), other countries in South East Asia (10.2%), and Eastern Europe (10.2%). Conclusions: The risk of a young person becoming infected with M. tuberculosis while living in Melbourne is very low. Our results do not indicate a need for the reintroduction of mass screening in Victorian schools. If targeted screening were to be considered, the group most likely to benefit would be recently arrived migrants from Indochina. |
Introduction |
Human infection with Mycobacterium tuberculosis is usually
clinically silent, and may then only be detectable by a positive
Mantoux skin test. A minority of infected individuals develop active
tuberculosis (TB), and those with pulmonary disease are the major
source of new human infections. For M. tuberculosis to
persist within a community over time, each person with pulmonary TB
must infect an average of 20 others.1,2 In developed countries, the
transmission rate is much lower than this, and the incidence of
tuberculosis has declined steadily for many years.2 In the United States, a 32-year trend of declining TB notifications was reversed in the mid 1980s.3 This resurgence is thought to have resulted in at least 51 000 unexpected cases of the disease, and has been attributed to the impact of AIDS, urban decay, homelessness and high levels of migration from regions where TB is endemic.4 Recent increases in incidence have also been reported from other developed countries, including Denmark, Italy, the Netherlands, Spain, Switzerland, France and the United Kingdom.5 In Australia, the incidence of TB has remained constant during the 1990s (rates per 100 000 population of 5.95 in 1990 and 5.75 in 1995).6 In Victoria, the annual incidence of TB per 100 000 declined from 47 in 1954 to 6.2 in 1992;7 it was 6.35 in 1995.6 In 1970 40% of new cases of active tuberculosis in Victoria were in people born overseas, but by 1990 this figure had risen to 80%. Currently, most new TB patients are migrants from Indochina and South East Asia.7 Although intending adult migrants are screened by chest x-ray before their arrival in Australia, migrant children are generally not screened, partly because childhood tuberculosis is not normally transmissible. In a recent inner-Sydney study, 27% of foreign-born Year 8 students (159 of 580), compared with 2% of those born in Australia (20 of 1221), had positive Mantoux reactions.8 As adolescents appear to have an increased risk (compared with children over three years and adults) of developing active tuberculosis,9 this group may become a source of new, locally acquired infection. During 1995, we conducted a Mantoux survey of healthy metropolitan Melbourne secondary students in Years 9 and 10. Our aim was to estimate the prevalence of asymptomatic TB infection, and to identify specific groups of students by region of birth who may benefit from future targeted screening and intervention programs. |
Methods |
The study was a cross-sectional survey of secondary school students
in the metropolitan region of Melbourne (population, 3.1 million,
with people aged 12 to 17 years comprising approximately 8%
[Australian Bureau of Statistics, 1996 Census]). To recruit enough
overseas-born students for estimating prevalence by region of
birth, we used a combination of targeted and random sampling, aiming
to include schools where at least 4% of students were born overseas,
plus a 5% random sample of all other schools.
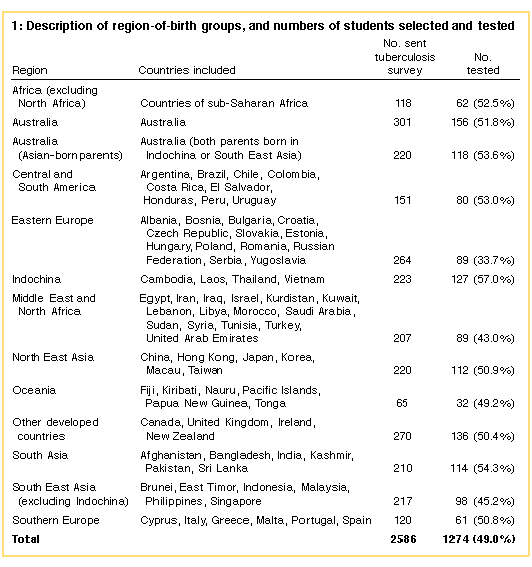
The Australian Bureau of Statistics (ABS) provided numbers and country of birth of Melbourne residents aged 12 to 17 years from the 1991 national census. The Directorate of School Education and the Catholic Education Office provided numbers of students at each school speaking a language other than English at home. These data were combined and used to select schools with high enrolments of overseas-born students. Independent schools were not included as they comprised only 18% of all Year 9 and Year 10 students (Directorate of School Education, personal communication), and we assumed that they would have a low proportion of recently arrived overseas-born students. Of 50 purposely selected and seven randomly selected schools approached, six and one, respectively, declined. The randomly selected school was replaced by a neighbouring school, so that 51 schools participated. Parents of all students in Years 9 and 10 at each participating school were provided with an explanatory letter and a short survey (in English and, if appropriate, one of 12 translations). The survey was part of a separate study on asthma, but included a question on country of birth and sought permission to approach students a second time for the TB study. The initial (asthma) survey was distributed to all 13 020 Year 9 and Year 10 students at the 51 schools; 9794 usable responses were obtained (75%). Respondents comprised 85% from purposely selected and 15% from randomly selected schools. From the returned surveys we created 12 notional region-of-birth groups, based partly on geography and partly on numbers of respondents to the first survey, and an additional group comprising Australian-born students with both parents born in Asia (Box 1). We aimed to enrol approximately 200 students from each group to allow us to estimate prevalence within each with a 95% confidence interval of ± 1.9% if the true prevalence were 2%, and ± 6.4% if the true prevalence were 30%. Selection for Mantoux testing was random, except when the number of respondents in a category was less than 200, in which case all respondents from that region were included.
|
| |
| Parents of students selected for Mantoux testing were sent
information letters, consent forms and a TB survey (in English and one
of 14 translations). This survey sought parental consent for Mantoux
testing and included questions on parents' country of birth, date of
arrival in Australia and history of BCG vaccination.
Mantoux testing (by two experienced nurses, with two assistants from the Victorian Tuberculosis Program) was performed at school. Responses on each TB survey were checked for completeness and each student's deltoids, forearms and scapulas inspected for the presence of BCG vaccination scars before testing, which involved intradermal injection of 0.1 mL of a 100 IU/mL solution of purified protein derivative (PPD; CSL Limited, Parkville, Vic.) to the volar aspect of the student's left forearm. A single batch of PPD was used throughout. At between 48 and 72 hours, the extent of transverse palpable induration was measured by ruler and recorded in millimetres. The study was approved by the Ethics in Human Research Committee of the Royal Children's Hospital. | |
Statistical analysis | We used the exact binomial method for confidence intervals and the chi-squared test for comparisons between groups. Logistic regression was used to estimate prevalences, adjusted for differences in duration of residence in Australia. As we had deliberately selected schools with high concentrations of foreign-born students, we estimated overall prevalence of infection by direct standardisation to the population distribution of region of birth in students aged 12 to 17 years in metropolitan Melbourne (1991 Census data, ABS). |
Results |
Of 2586 students (the results of our efforts to create the 13 groups)
sent the TB survey, 620 (24.0%) did not return it, and 692 (27%)
returned completed surveys but declined to be tested or were away on
the day of testing. Test results were therefore available for 1274
students (49%).
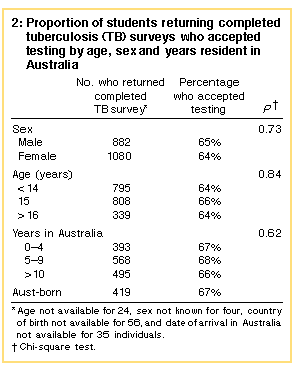
To explore the potential for response bias, survey response rates and acceptance of testing were compared between subgroups defined by age, sex and time since arrival in Australia (Box 2). Younger students and females were statistically more likely to return their surveys than older students and males (data not shown), but refusal to be tested did not vary between these subgroups.
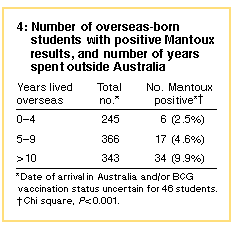
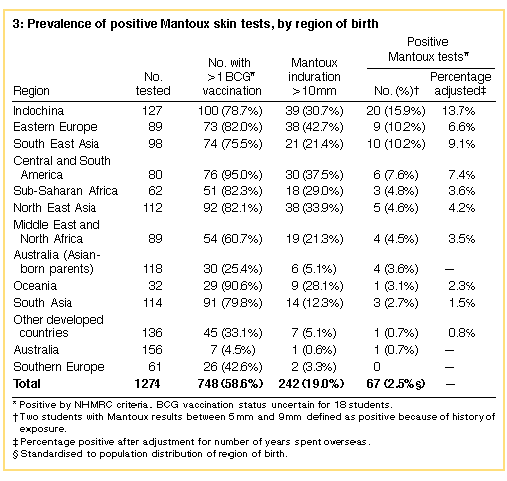
Proportions of students with Mantoux reactions that were positive by National Health and Medical Research Council criteria (≥5 mm with a history of recent exposure; ≥10 mm and no prior BCG vaccination; ≥15 mm and prior BCG vaccination8,10 ) were compared by region-of-birth group (Box 3). Birth overseas, number of years resident outside Australia (Box 4) and past BCG vaccination were predictors of a positive result. The crude prevalence was 5.3%.
|
| |
After standardisation by region of birth, we estimated that 2.5% of all students in Years 9 and 10 in metropolitan Melbourne had positive Mantoux results (95% CI, 1.1-3.9%). Students born in Australia, "other developed countries" and Southern Europe had the lowest rates (0.7%, 0.7% and 0, respectively). The highest rates were observed in students born in Indochina (15.9%), other countries in South East Asia (10.2%), and Eastern Europe (10.2%) (Box 3). Differences in mean number of years resident overseas between the groups prevented direct statistical comparison, so logistic regression was used to estimate the odds ratio for increase of risk for each year lived overseas (odds ratio per year, 1.15; 95% CI, 1.08-1.24), and to standardise rates by region so they could be compared directly. After this adjustment, statistically significant differences in prevalence by region persisted, but the ranking of some regions, most notably Eastern Europe, was altered (Box 3).
Prevalence of TB in the group of Australian-born students with Asian-born parents was 3.6%. None of these students had received a prior BCG vaccination. This prevalence was fivefold higher than the background rate of 0.7% for other students born in Australia, but this difference did not reach statistical significance (P = 0.17, two-tailed Fisher's exact test). The 244 students with a result ≥10 mm and the two with a result ≥5 mm plus a history of exposure to someone known to have TB were referred to a special clinic at the Royal Children's Hospital for chest x-ray and clinical review. However, 10 students with indurations ≥10 mm declined to attend the clinic, five of whom had positive Mantoux reactions. Of the 236 students who attended the clinic, 174 had at least one BCG vaccination scar and/or documentary evidence of BCG vaccination and a Mantoux reaction < 15 mm; these were therefore considered to have negative results. Students with results positive by NHMRC criteria8,10 were offered isoniazid preventive therapy, provided there was no evidence of active disease and they had not been previously treated. Sixty-two students had positive Mantoux reactions. Of these, seven had previously been prescribed isoniazid preventive therapy, two had previously been treated for TB disease, and one (a recently arrived refugee from East Timor) had active pulmonary TB. Five students refused isoniazid therapy, and five were not offered therapy as they were considered to have a reduced risk (recent BCG). Forty-two students with positive Mantoux reactions were offered isoniazid preventive therapy; 38 (90%) completed six months of treatment. Multiple BCG scars (evidence of previous vaccinations) were common in students from Eastern Europe (mean number, 1.6; 33% of students with ≥2 scars) and the Middle East, whereas those from Indochina generally had only one (mean number, 0.9; 11% of students with ≥2 scars). | |
Discussion |
The risk of a young person becoming infected with M. tuberculosis
while living in Melbourne appears to be very low.
The major determinant of the size of the Mantoux reaction in this study was birth overseas. However, most students with reactions to testing had also received at least one prior BCG vaccination, which complicates interpretation. Most Australian-born students had not been vaccinated, and the prevalence of infection in this group was very low (0.7%, giving a calculated annual risk of infection of 0.04% per year). The higher prevalence in Australian-born students with Asian-born parents may be the result of low-level transmission within migrant communities, although the apparent difference could have been due to chance. For those born overseas, the number of years spent outside Australia correlated positively with Mantoux results, indicating that the risk of infection increases with duration of residence in an endemic region. The prevalence of infection in Year 9 and Year 10 students in metropolitan Melbourne appeared to be approximately half that identified in a recent survey in Year 8 students in inner Sydney, both overall and within specific migrant subgroups.8 The authors of the Sydney study commented that the prevalence they identified was higher than previously reported in Australia and have since found a slightly lower prevalence in a further survey of younger students.11 Our lower rate may be the result of differing patterns of migration between Melbourne and Sydney, or the selection of our sample from the whole metropolitan region instead of just the inner city. Although only half of eligible students in our study were tested, we do not believe that our results are systematically biased in a way that would have led us to grossly underestimate prevalence. In particular, we found no association between recent arrival in Australia and the likelihood of refusing to be tested, and the group with the lowest participation rate (Eastern Europe) showed the second-highest prevalence. In retrospect, acceptance of testing may have been improved by selecting whole classes rather than individuals within a class for testing, and our two-stage study design allowed ample opportunity for students to withdraw. However, any future targeted screening program would need to select individuals from within a larger group, and part of our study rationale was to investigate the acceptability of such programs. Even if it were assumed that non-participants had twice the prevalence of those tested, the true prevalence would only be 50% greater than our estimates. There is controversy about the influence of BCG vaccination on the results of subsequent Mantoux testing. In countries with a high prevalence of TB infection, a single BCG vaccination is often given shortly after birth, but this is unlikely to influence the result of a Mantoux test 15 years later.12 By contrast, BCG given to older children or given several times during childhood probably does influence Mantoux reaction size.13 Although the current NHMRC guidelines make some allowance for past BCG vaccination, they could be further refined.13 For example, for recently arrived migrants who have received a single BCG vaccination early in life and who have lived for many years in a region of high prevalence it may be appropriate to use ≥10 mm to indicate a positive reaction, while for migrants from countries that routinely give three BCG vaccinations in childhood but have a lower prevalence of infection ≥20 mm may be more appropriate.13 Whether or not such refinements are introduced, the distinction between positive and negative reactions will remain somewhat arbitrary; a more reliable test is urgently required. Mass screening of secondary school students by Mantoux test was discontinued in Victoria over 10 years ago. Our results do not suggest that such programs need to be reintroduced, and recent overseas studies suggest that mass screening at school is unlikely to be cost effective.14-16 However, 24% of overseas-born students in our study had Mantoux reactions ≥10 mm, one of whom had active pulmonary disease and one-quarter of whom were considered eligible for isoniazid preventive therapy. If reactions of ≥10 mm for students born in a high-risk region with a history of having received a single BCG vaccination in infancy were considered positive, the number of students eligible for preventive therapy would increase further. If targeted screening were introduced, the group most likely to benefit would be recently arrived students from Indochina. We offered isoniazid therapy to students who tested positive because adolescents have an increased risk of developing active tuberculosis.9,17 However, we were mindful that isoniazid therapy is not entirely without risk even in young people,18-20 and that the risk of infection progressing to disease in an affluent society with a low prevalence of HIV infection may be much lower than the 10% often quoted.21 We cannot therefore be completely confident that wider use of isoniazid in this way would result in a net benefit to the Australian community. |
Acknowledgements | This study was supported by a grant from the John Burge Estate administered by the Victorian Department of Human Services. We gratefully acknowledge the assistance of the principals, coordinators, teachers, students and parents at the participating schools. We also wish to thank the following individuals: Mary Randall, Mary McColl and staff of the Victorian Tuberculosis Program; Marita Dalton, Colin Powell, Department of Thoracic Medicine; and Susan Sawyer, Centre for Adolescent Health, Royal Children's Hospital. |
References |
|
Authors' details
Clinical Epidemiology and Biostatistics Unit, Royal Children's Hospital, Melbourne, VIC.Paul D R Johnson, FRACP, PhD, Research Officer, Royal Children's Hospital Research Institute (also, Infectious Diseases Physician, Department of Infectious Diseases and Clinical Epidemiology, Monash Medical Centre);
John B Carlin, BSc(Hons), PhD, Deputy Head (also, Associate Professor, University of Melbourne, Department of Paediatrics);
Catherine M Bennett, BSc(Hons), Research Officer;
Jane Hulls, RN, Research Nurse;
Terry M Nolan, PhD, FRACP, Head (also, Associate Professor, University of Melbourne, Department of Paediatrics).
University of Melbourne, Department of Paediatrics, Royal
Children's Hospital, Melbourne, VIC.
Peter D Phelan, MD, FRACP, Stevenson Professor, and Head
(currently, Emeritus Professor of Paediatrics).
Department of Microbiology and Infectious Diseases, Royal
Children's Hospital, Melbourne, VIC.
Michael Starr, MB BS, FRACP, Paediatrician.
Reprints: Dr P D R Johnson, Department of Infectious Diseases
and Clinical Epidemiology, Monash Medical Centre, Clayton, VIC
3168.
E-mail: Paul.Johnson AT med.monash.edu.au
Readers may print a single copy for personal use. No further
reproduction or distribution of the articles
should proceed without the permission of the publisher. For
permission, contact the
Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
Received 4 March 2026, accepted 4 March 2026
- Paul D R Johnson1
- John B Carlin
- Catherine M Bennett
- Peter D Phelan
- Michael Starr
- Jane Hulls
- Terry M Nolan