Apolipoprotein screening in Australian children: feasibility and the effect of age, sex, and ethnicity
Judith F Lynch, Michelle D Marshall, Xing L Wang and David E L Wilcken
MJA 1998; 168: 61-64
Abstract -
Introduction -
Methods -
Results -
Discussion -
References -
Authors' details
-
-
©MJA1998
Abstract |
Objectives: (i) To evaluate the feasibility of
detecting adverse lipid profiles in schoolchildren by measuring
capillary dried blood spot apolipoprotein levels, and (ii) to assess
the effect of age, sex and ethnicity on apolipoprotein levels. Design: We measured capillary dried blood spot apolipoproteins B and A-I (apo B and apo A-I); assessed levels in relation to age, sex and ethnicity; and recalled children with elevated levels for a full lipid profile measurement. Participants and setting: 6992 children (3501 boys and 3491 girls), aged 5-13 years, from schools in eastern Sydney, 1991-1995. Main outcome measures: Capillary blood levels of apolipoproteins B and A-I, and serum total cholesterol level. Results: Of the 6951 children who provided an adequate fingerprick blood sample, we recalled 1465 children (21.1%) (640 boys [43.7%] and 825 girls [56.3%]) with elevated apo B levels and/or apo B : apo A-I ratios for further testing, either by us or by their family doctor (overall estimated compliance rate up to 70%). Among the 458 children who returned to us, there was a 90% positive predictive value for a total cholesterol level of over 4.5 mmol/L in those with both elevated apo B levels and high apo B : apo A-I ratios. Girls had higher apo B levels and apo B : apo A-I ratios than boys (P < 0.00001 for both), and in both sexes there was a trend downwards for apo B and upwards for apo B : apo A-I ratio over the age range tested, but levels were relatively stable between the ages of 6 and 10 years. Indian children (1.5% of the screened population) had the highest apo B levels, followed by white children (71.1%); Asian children (9.2%) had the lowest (P < 0.00001 compared with Indian and white children). Conclusions: The high positive predictive value of capillary blood apolipoprotein levels for an adverse lipid profile in children suggests that measuring apolipoprotein levels by this method is a useful initial approach to cardiovascular risk assessment. |
Introduction |
Despite a recent decline in cardiac deaths, coronary disease is still
the largest single cause of premature death in Australia.1 It is known that atherogenesis, the
underlying pathological process, may begin in childhood;2,3 that relevant risk factors may
track from childhood to adulthood (particularly elevated levels of
total and low-density lipoprotein [LDL] cholesterol and increased
body weight); and that these factors tend to aggregate within
families.4,5 With these
considerations in mind, we explored the feasibility of screening for
adverse lipid profiles in a target group of primary schoolchildren,
with the aim of implementing family-based coronary prevention by
secondarily identifying any parents at risk.
We measured levels of apolipoprotein B (apo B), the carrier protein for the atherogenic LDL cholesterol, and apolipoprotein A-I (apo A-I), the principal carrier protein for the antiatherogenic high density lipoprotein (HDL) cholesterol, in capillary dried blood spots from 6992 primary schoolchildren aged 5-13 years. There is increasing evidence that high apo B and low apo A-I levels are as reflective of cardiovascular risk as are their respective lipoproteins.6 We describe here the positive predictive value of high apo B and/or apo B : apo A-I ratio for elevated total cholesterol levels and the effects of age, sex and eth nicity on lipid levels in Australian children. |
Methods |
School and population demographics
Blood collection and apolipoprotein measurement
Recall
We notified the parents of the children for recall by letter, explaining the results and suggesting that both parents, the child, and any other siblings might come to us for further lipid testing, or attend their family doctor. In those who returned to us, we measured total cholesterol level, as well as HDL cholesterol and triglyceride levels, in a venous blood sample by standard methods. LDL cholesterol level was calculated by the Friedewald formula,10 and we also measured serum apo B, apo A-I and Lp(a) by the methods outlined above.7-9 The parents of children with apo B levels and apo B : apo A-I ratios below the established cut-off points were also sent a letter explaining that their children's levels currently fell below the recall criteria. These families were sent a healthy lifestyle pamphlet specifically directed to maintaining a low level of cardiovascular risk; they were also asked if they would like to volunteer as a healthy control family and have complete lipid profiles measured as above. Statistical analysis
Ethical approval
|
Results |
Initial testing
Recall population
In all, 458 of the children recalled returned with their families to our laboratory for lipid studies (31.2% compliance rate). To obtain an assessment of how many may have been seen by their family doctor (the other option we suggested), we contacted 205 of the remaining 1007 families. Among these, 118 had seen their family doctor for follow-up, and 89 had either moved or were not interested in further testing. This suggested an overall compliance rate of about 70%, if this sample was representative. Of the non-recalled "normal" population of 5486 (78.9%), only 151 families (2.7%) volunteered for lipid testing, an insufficient number for meaningful statistical analysis. Predictive value
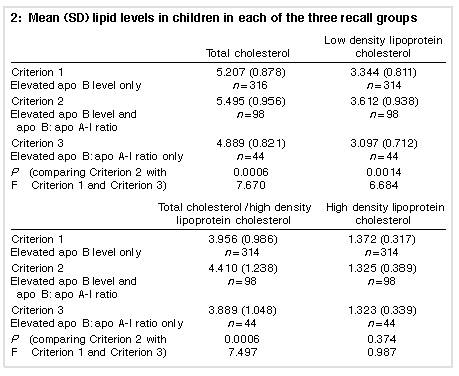
Raising the cut-off point did not substantially improve the positive predictive value of our screening method. When the cut-off point for apo B was increased from 0.550 g/L to 0.700 g/L, for example, the positive predictive value of Criterion 2, the most strongly predictive, only increased to 94% (95% CI, 91%-97%). ANOVA confirmed the association between high apo B levels from analysis of dried blood spot and an adverse lipid profile, and also that a combined high apo B level and apo B : apo A-I ratio was a stronger predictor. In this analysis, criteria established by high apo B, high apo B : apo A-I ratio and a combination of both were determinant categorical variables, and the total cholesterol and LDL cholesterol levels and total chol es terol to HDL cholesterol ratio were the continuous outcomes. Total (P < 0.0006) and LDL cholesterol (P < 0.0014) levels and the total cholesterol to HDL cholesterol ratio (P < 0.0006) were all significantly higher in Criterion 2 than in Criterion 1 or Criterion 3 (Table 2).
Sex, age and ethnicity
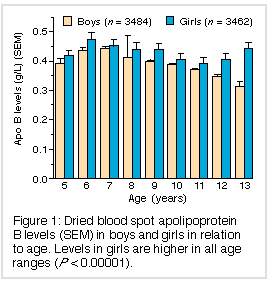
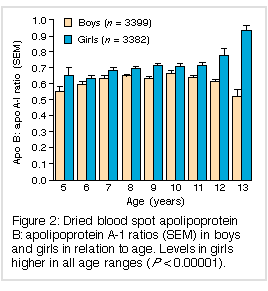
As shown in Figures 1 and 2, there was a downward trend in apo B level and an upward one in apo B : apo A-I ratio in boys over the age range screened, while the opposite was observed in girls; but levels were relatively consistent in both between the ages of 6 and 10 years.
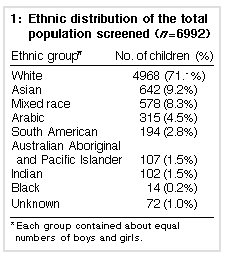
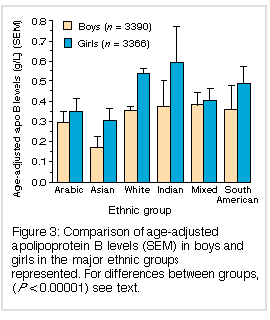
Most of the 6951 children screened were white (71.1%); the remainder were of diverse ethnic origins (Table 1). The mixed-race group (8.3% of the total) included white/Asian (28.2%), white/Arabic (17.8%), white/South American (10.7%), and other combinations (56.7%). As age was a significant contributor to apo B concentrations, we compared the "age adjusted apo B levels" for both boys and girls to assess potential differences between major ethnic groups (Figure 3). Indian children had the highest apo B levels, followed by white children. Asian children had the lowest levels and these were significantly different from those of the Indian, white, mixed-race and South American children (P < 0.00001 for each). We did not include black, Australian Aboriginal or Pacific Islander ethnic groups in this analysis because of small numbers and highly skewed age distributions. In all ethnic groups, girls had higher levels than boys (Figure 3). |
Discussion |
We have established previously that apolipoprotein measurements
provide a convenient and effective approach to the detection of
dyslipidaemia,14 and that
apo B levels in children are correlated with the occurrence of
coronary events in their grandparents, which highlights the
relevance of measurements in children to assessing risk of vascular
disease in older family members.15
We have also established a clear-cut association between
increased apo B levels and apo B : apo A-I ratios and body mass index in
Australian children.16
Here, we extend these find ings by demonstrating the feasibility of
screening of schoolchildren's capillary blood apolipoprotein
levels as an approach to family-based primary coronary prevention.
Although we do not have adequate data to determine the sensitivity or specificity of our screening method, the presence of both an elevated apo B level and raised apo B : apo A-I ratios had a positive predictive value of 90% for the detection of elevated total serum cholesterol level. Raising the apolipoprotein cut-off points only improved the predictive value to 94%. This method identified elevated apolipoprotein levels in 21.1% of our population, of whom 90% had elevated total cholesterol levels. Screening for apolipoprotein levels may be more relevant than screening for total cholesterol levels alone, as total cholesterol level does not show the interrelations between levels of the atherogenic LDL and the antiatherogenic HDL cholesterol.17 In a small number of children (0.48%) apo B levels were elevated without elevation of total cholesterol level.18 These families were also provided with dietary and lifestyle advice as this may also be associated with increased cardio vascular risk. Our findings clearly show highly significant differences between boys and girls at this age, with girls having higher apo B levels and apo B : apo A-I ratios than boys. We suspect this biological difference, which was evident before the onset of puberty in both boys and girls, to be hormonally based, although we have no data to support this. The vari ation in apolipoprotein levels within the age range studied could also be hormonally based. Whatever the mech anisms, these same age-related sex differences have been identified in several other studies in children.19,20 The consistency of these results clearly indicates a need to have different cut-off points in boys and girls for elevated apolipoprotein levels. Our results also define the age range (between 6 and 10 years) when levels are most stable and most appropriate for screening, findings which will be incorporated into future studies. There were uniform sex-related differences in apolipoprotein levels in each of the racial groups in our population, as well as very significant differences in levels between the various ethnic groups, as has also been found in previous studies.21,22 In our population, Indian and white children had the highest apo B and apo B : apo A-I ratios and Asian children had the lowest. It is well established that Chinese and Japanese populations have much lower cholesterol levels than whites and a correspondingly lower prevalence of coronary artery disease. Indian populations have a higher prevalence of cardiovascular disease.23 While these variations may relate to the genetic background of each ethnic group, diet and lifestyle undoubtedly make major contributions, and this is particularly evident in non-Western groups who have moved to live in a Western society. The higher prevalence of coronary disease in Indians living in the United Kingdom, for example, is well documented.24 Ethnic differences may become blurred with time if dietary habits become more uniform, and this has occurred among Asians emigrating to the United States and the United Kingdom; within a generation they acquired local lifestyles and a correspondingly higher prevalence of coronary artery disease than that in the communities they had left.25,26 In conclusion, our study demonstrates the feasibility of conducting a program of measuring apolipoprotein levels in capillary blood samples to assess lipid profiles in children to facilitate family-based coronary prevention. It documents wide acceptance by both schools and parents. Implicit in such a study is the potential for a multiplier effect in that, if a child has an elevated apolipoprotein level and therefore an adverse lipid profile, it is very likely that this will also be seen in at least one parent and other siblings.27 Our ongoing screening program requires the establishment of a concurrent intervention program to improve dietary and lifestyle habits of affected children and their parents. This is of more immediate relevance to affected parents as they are approaching the age of overt coronary disease. However, the program provides an ideal opportunity to establish healthy lifestyles in young children at a time of easy acceptance, and with the potential for preventing a disorder that may have its origins in childhood.28 |
References |
|
Authors' details
Department of Cardiovascular Medicine, Prince Henry and Prince of Wales Hospitals, Sydney; and Community Health Services and Programs, South Eastern Sydney Area Health Service, Royal South Sydney Hospital, Sydney, NSW.Judith F Lynch, HTech, Heart Health Education Program Co-ordinator;
Michelle D Marshall, BSc, Scientific Officer;
Xing L Wang, PhD, Research Fellow;
David E L Wilcken , MD, FRACP, Visiting Professor of Medicine.
Reprints will not be available from the authors.
Correspondence:
Professor D E L Wilcken, Department of Cardiovascular Medicine, Room
163, Clinical Sciences Building, Prince Henry Hospital, Little Bay,
Sydney, NSW 2036.
Readers may print a single copy for personal use. No further
reproduction or distribution of the articles
should proceed without the permission of the publisher. For
permission, contact the
Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au>".
<URL: http://www.mja.com.au/>
Received 4 March 2026, accepted 4 March 2026
- Judith F Lynch
- Michelle D Marshall
- Xing L Wang