Evaluation of the ThinPrep Pap test as an adjunct to the conventional Pap smear
Jennifer M Roberts, A Marion Gurley, Julia K Thurloe,
Ronald Bowditch
and Colin R A Laverty
For editorial comment, see Wain
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au/>".
Abstract - Introduction - Methods - Results - Discussion - Acknowledgements - References - Authors' details
- - ©MJA1997
Abstract |
Objective: To evaluate the ThinPrep Pap test as an
adjunct to the conventional Pap smear. Design and setting: Prospectively collected cervical samples were split for independent screening at a large specialised private gynaecological pathology practice in Sydney. Main outcome measures: Detection of additional significant abnormalities (cervical intraepithelial neoplasia 1, or more severe); changed management recommendations from "repeat smear in 12 months" or "...six months" to "colposcopy"; a reduction in unsatisfactory reports. Results: 35 560 paired (split-sample) conventional and ThinPrep slides were prepared. Significant abnormalities were detected in 724 conventional smears (2%). Additional significant abnormalities were found in 85 ThinPrep slides whose corresponding conventional smear was negative or unsatisfactory even after review, representing a 12% increase in the detection of significant abnormalities. As a result of the addition of ThinPrep, management recommendations were changed from "repeat smear in 12 months" or "...six months" to "colposcopy" for 89 of 1669 women whose conventional Pap smears showed minor non-specific changes or papillomavirus. There were 1258 conventional smears (3.5%) that were unsatisfactory compared with 235 ThinPrep slides (0.7%); for only 74 samples (0.2%) were both slides unsatisfactory. Conclusions: The addition of the ThinPrep Pap test improves detection and clinical management of cervical abnormalities, and reduces the number of unsatisfactory samples which would otherwise require repeat tests. |
Introduction |
The conventional Pap smear has been the mainstay of screening for
cervical precancer for approximately 50 years, without major
changes in techniques relating to preparation and interpretation.
In recent years there has been increasing pressure to improve Pap
smear standards to reduce the occurrence of false negative results,
with their associated morbidity and mortality for the women
involved, and legal consequences for health professionals.1,2 Many false negative cases are the result of problems of sample transfer and smear quality.3,4 The conventional Pap smear represents only a small subsample of the cellular material collected on the sampling implement,5,6 with the remainder traditionally being discarded. Importantly, this small subsample is a non-random fraction of the material collected and is not necessarily representative of the whole. The traditional smearing technique also produces slides which vary greatly in quality, making it sometimes difficult to find and interpret abnormal cells. Improvements in both subsample selection and slide quality should therefore reduce the number of false negative cases. The ThinPrep Pap test is an automated technique developed with the aim of producing better quality slides.7 Each cervical sample is rinsed into a vial of fixative fluid. In the laboratory the sample is homogenised, and a representative subsample is collected on a single-use filter, transferred to a glass slide, and stained in the routine fashion. This technique results in a better quality preparation, as the problems of poor fixation, uneven thickness of the cellular spread, and obscuring of cells by blood and inflammatory exudate are overcome. We aimed to evaluate the impact of this technology on detection rates of significant abnormalities, accuracy in reporting, and numbers of unsatis - fact ory preparations when it was used in addition to the conventional Pap smear. |
Methods |
In a pilot study in the first half of 1996, we evaluated 1051 samples to
test the performance of the ThinPrep 2000 machine (Cytyc
Corporation, Boxborough, Mass., USA), as previous experience with
an earlier model had highlighted some technical problems.8 The pilot study showed slides of
uniformly high quality and so, in July 1996, the test was offered to all
referring practitioners at a charge to patients of $20.30.
For this study, we analysed paired samples collected between 1 July 1996 and 30 May 1997 by practitioners who chose to offer ThinPrep (TP) to their patients as an adjunct test. We used a split-sample protocol in which routine samples of the transformation zone (squamo-columnar junction) -- the area of the cervix where abnormalities are most likely to occur -- were taken with a broom-like device, the Cervex brush (Rovers BV, Netherlands). We insisted on the use of this implement as our earlier study8 had shown that sampling with a spatula was more likely to result in a TP slide with insufficient cells. For postmenopausal women and those who had had previous cervical surgery or ablation, an endocervical brush sample was added. After a conventional Pap smear was made, the sampling implement was vigorously rinsed in a vial of PreservCyt fixative fluid (Cytyc Corporation, Boxborough, Mass., USA). TP slides were prepared from the vial by the ThinPrep 2000 machine and both slides were stained routinely. A duplicate of each request form was made, and the conventional smear and the TP slide were examined independently by different cytotechnologists. Nineteen of our 28 cytotechnologists were involved in reading the TP slides; all 28 read conventional smears. Any slide showing minor abnormalities was reviewed by a second experienced cytotechnologist. If cervical intraepithelial neoplasia (CIN) of any grade was detected, or if a high grade lesion was suspected, the case was referred to a cytopathologist for review. Slide pairs (conventional Pap smear and TP) displaying a significant discrepancy were also reviewed. The final report issued to the referring doctor was structured according to the National Health and Medical Research Council (NHMRC) reporting terminology9 (see Box 1) and contained any relevant information obtained from both slides, together with the relevant management recommendations. For the purposes of analysis, separate final results of the two slides were also recorded. A significant abnormality was defined as one for which the management recom mendation is "colposcopy" (see Box 1). This includes any grade of CIN, adenocarcinoma in situ (AIS), invasive carcinoma or the suspicion of a high grade abnormality ("inconclusive" in NHMRC terminology). |
Results |
Approximately 500 referring practitioners chose to offer TP to their
patients as an adjunct test. Some of these doctors offered the TP test
to all women, and others only to selected women. Thirty-five per cent
of women having non-screening cytological tests had TP, whereas only
24% of women having routine cytological tests had TP. Currently,
about 30% of all smears received in this laboratory are accompanied by
a TP sample.
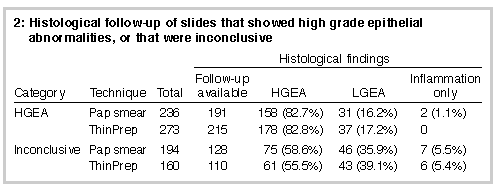
We received 35 560 paired split-sample conventional Pap smear slides and TP samples for analysis in this study. Box 1 presents a comparison of final (i.e., reviewed) results for TP and conventional Pap smear slides. Of the 34 141 paired results that were satisfactory for both slides, 32 195 (94.3%) showed total agreement and 1946 (5.7%) did not. Of the latter group, the TP slide showed a more severe abnormality than the conventional slide in 1194 (61.4%), and the opposite was true in 752 (38.6%; chi-squared = 100.4; df = 1; P < 0.001). For the 1946 paired slides which did not show total agreement, 271 had substantial discrepancies in which colposcopy would have been recommended based on the result of one test, but not the other. Of these, colposcopy would have been recommended on the basis of the TP result alone for 167 (61.6%), and on the basis of the conventional Pap smear result alone for 104 (38.4%; chi-squared = 14.6; df = 1; P < 0.001). Significant abnormalities were detected in 724 conventional smears. An additional 85 significant abnormalities were detected on TP slides for which the corresponding conventional slides, even after review, were either negative (78) or unsatisfactory (7). This represents a 12% increase in the detection of significant abnormalities. There were 14 TP slides that predicted high-grade epithelial abnormality (HGEA), while the corresponding conventional smears were either negative or unsatisfactory. Histological follow-up of 11 of these confirmed HGEA in eight, and low-grade epithelial abnormality (LGEA) in three. There were also 27 TP slides reported as inconclusive, while the corresponding conventional smears were either negative or unsatisfactory. Histological follow-up of 17 of these showed HGEA in seven, LGEA in eight, and inflammation in only two. Further, 15 conventional smears originally reported as negative, and reviewed because their TP counterparts showed an abnormality, were subsequently reported as HGEA or inconclusive. These "screening" false negatives are not represented in Box 1, which shows only final results (i.e., after review). ThinPrep slides not only increased detection of abnormalities, but also influenced management recommendations. On the basis of conventional smear tests, the recommendations for 1669 women (4.7%) with minor non-specific changes or features of human papillomavirus (HPV) effect were "repeat smear in 12 months" or "...six months", respectively. For 89 of these women (5.3%), this recommendation was changed to "colposcopy" as a result of a higher grade abnormality being detected on the TP slide. Histological follow-up concentrated on the predicted HGEA and inconclusive categories. Findings are shown in Box 2 (below).
|
|
Of conventional smears, 1258 (3.5%) were found to be unsatisfactory,
but only 74 (0.2%) were unsatisfactory by both methods, representing
a 94% reduction in unsatisfactory reports. TP predicted HGEA in four
smears reported as unsatisfactory on the conventional smear; all
four have been histologically confirmed to be HGEA.
Minor abnormalities (minor non- specific changes/HPV) were detected in 1669 (4.7%) of the conventional smears and 2027 (5.7%) of the TP slides. An endocervical component was absent in 8.3% of the conventional smears and in 20.0% of the TP slides. In 6.4% of cases, neither slide displayed an endocervical component. With respect to glandular abnormalities, 13 cases of adenocarcinoma in situ (AIS) or adenocarcinoma were detected by both methods. There was one histologically confirmed case of adenocarcinoma in which the conventional smear was reported as inconclusive (suspicious of AIS) and the corresponding TP slide was negative. Further TP slides were made from the remaining sample in the vial but none of these contained abnormal cells. | |
Discussion |
Overall, we found that diagnostic agreement between the
conventional Pap smear and the TP Pap test was high. Where there was
disagreement, a higher grade abnormality was predicted
significantly more often by TP than by the conventional smear.
The addition of the TP test resulted in detection of additional histologically confirmed HGEA, leading to a reduction in the number of false negatives and a corresponding increase in sensitivity. The fact that the same proportion of HGEA (83%) was histologically confirmed for both conventional Pap smear and TP shows that TP does not result in undue overreporting, and that, although sensitivity has been increased, the positive predictive value of the test has been maintained. The extra significant abnormalities detected on TP and the increased accur acy of reporting are mainly attributable to improved subsampling of the cervical specimens, resulting in fewer "subsampling" false negatives. Computer-assisted rescreening of conventional smears would have no impact on this area. "Screening" false negatives (although few in this study) will also be reduced by the addition of a slide of much higher quality, and by the independent screening of additional material. That there were cases in which an abnormality detected on conventional smear was not detected on TP is not surprising, as the split-sample protocol favours the conventional smear. In these cases it appears that all abnormal cells in the sample were transferred to the conventional smear, leaving none in the TP vial. A "direct-to-vial" protocol in which 100% of the material collected is rinsed into the fluid fixative should eliminate this problem. However, if only a very small number of abnormal cells are present in the vial, then they may not be represented on any single TP slide. The addition of the TP test resulted in a large reduction in reporting of unsatisfactory preparations. This is a direct result of removal of the many variables associated with the conventional Pap smear technique (poor fixation, uneven thickness of the cellular spread, and obscuring of cells by blood and inflammatory exudate), and represents significant time and cost savings, as the need for a repeat smear is avoided. Significantly, there were four women with histologically confirmed HGEA which was predicted by TP, but whose conventional smears were reported as unsatisfactory. These abnormalities may have been detected by the recommended repeat smear three months later, but there is always a risk that women will not return for follow-up. A very small percentage of TP slides were unsatisfactory and this was always the result of there being insufficient cells in the specimen. In these cases the machine reported a dilute specimen. We believe that a number of these cases were the result of the use of incorrect sampling implements, contrary to the recommended protocol. Of some surprise to us was the increase in reporting of minor non-specific changes. While some of this no doubt represents a genuine increase in detection of minor abnormalities, we feel that other factors must be contributing. In particular, we believe that the improved nuclear morphology obtained in the TP process requires cytotechnologists and cytopathologists to "relearn" subtle criteria used at this end of the diagnostic spectrum. We noted this trend early in the study period and have been addressing it with numerous educational sessions. We continue to monitor reporting in this category. The addition of TP tests resulted in a decreased proportion of combined reports lacking an endocervical compon ent. However, TP alone had a higher rate of absent endocervical compon ent. The reason for this is unclear, but may relate to TP slides being prepared from left-over cellular material. We can only speculate that the "endocervical component present" rate may improve in a direct-to-vial situation. Even though there were more TP slides lacking an endocervical component, there was only one case in which there was a significant glandular abnormality present in the conventional smear and not in the TP slide. As further slides prepared from the TP vial also failed to show abnormal cells, we assume that no abnormal cells were present in the vial. An important advantage of the TP process is the presence of further cellular material in the fluid fixative which can be used to prepare more slides, or for HPV typing which may have clinical relevance in the triage of patients with low grade abnormalities.10 In the United States, the Food and Drug Administration has approved the TP Pap test as a replacement for the conventional Pap smear.11 This decision was based on analysis of data on 7360 paired samples derived from six different centres (Data on file, Cytyc Corporation, Boxborough, Mass., USA). Our data, on a larger sample from a single practice, support the assertion that the TP Pap test performs substantially better than the conventional smear on analysis of significant parameters. While we continue to offer the TP Pap test as an additional proced ure, we anticipate that the machine-made slide may replace the conventional smear in the future. |
Acknowledgements |
We thank Tabatha Lovelace, Lisa Wong, Rozanne Van Gramberg and Samira
Bounassif for technical assistance.
Statement of potential conflict of interest: The Cytyc Corporation (Boxborough, Mass., USA) lent us one of the Thinprep 2000 machines used in this study. The company was not involved in the design of the study, collection of data, analysis of results or preparation of the manuscript. |
References |
(Received 3 Feb, accepted 14 July 1997) |
Authors' details
Dr Colin Laverty & Associates, Pathologists, Eastwood, NSW.Jennifer M Roberts, MB BS, FRCPA, Pathologist;
A Marion Gurley, MB ChB, FIAC, Pathologist;
Julia K Thurloe, BSc, MEc, Statistician;
Ronald Bowditch, BScAg CT(ASC), Senior Cytotechnologist;
Colin R A Laverty, MB BS, FRCPA, Principal Pathologist.
Reprints: Dr C R A Laverty, Dr Colin Laverty & Associates, Pathologists, 18 Glen Street, Eastwood, NSW 2122.
©MJA 1997
<URL: http://www.mja.com.au/>
© 1997 Medical Journal of Australia.
Received 4 March 2026, accepted 4 March 2026




