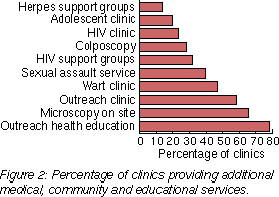Evaluation of sexual health services within Australia and New Zealand
Caron Marks, Robin L Tideman and Adrian Mindel
MJA 1997; 166: 348-352
For comment see Fairley
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au/>".
Abstract - Introduction - Methods - Results - Discussion - Acknowledgements - References - Authors' details - Figure 1 - Figure 2
- - ©MJA1997
Abstract |
Objective: To examine and compare specialised
services for patients with sexually transmitted diseases (STDs) in
Australia and New Zealand. Design: Postal questionnaire survey. Participants and Setting: All STD facilities in Australia and New Zealand in 1993. Main Outcome Measures: Patient numbers and demography; staffing levels; the role of nurses; diagnostic and treatment protocols; contact-tracing policies; and the availability of specialist medical services and community and education programs. Results: 83 of 100 clinics responded; 52 were urban, 21 rural, and nine remote. 95% were open to men and women. Staffing levels were similar in Australia and New Zealand and there was considerable consistency in diagnostic techniques and treatment among clinics. Australian clinics more often used ciprofloxacin or ceftriaxone as the treatment of first choice for gonorrhoea; New Zealand clinics were more likely to test for Chlamydia using direct immunofluorescence; and Australian clinics were more likely to test for hepatitis A and offer hepatitis B vaccination to a broader range of patients. 88% of clinics always traced contacts for gonorrhoea, 86% for syphilis and 77% for Chlamydia . 98% of clinics offered HIV test counselling, and 78% STD health education. Conclusions: The number of sexual health services has increased over the past decade. Other improvements include most clinics being open to both men and women, and consistency in the diagnosis, treatment and contact tracing of STDs. However, given the lack of adequate comparative data and the variable quality of national surveillance data, it is difficult to determine whether current facilities are meeting service needs. |
Introduction |
The control of sexually transmitted diseases (STDs) depends on
health promotion, education, the provision of adequate facilities
for the diagnosis, treatment and contact tracing of STDs, and ongoing
research.1 One of the more
comprehensive national STD services (involving more than 200
standardised clinics) is in the United Kingdom.2,3 By contrast, Australia currently
has a fragmented STD control system in which each State and Territory
works independently and with different health policies. There are no
nationally agreed guidelines for the diagnosis, treatment and
tracing of contacts of patients with STDs,4 availability of different tests
varies across the continent, and treatment protocols may vary
according to antibiotic resistance patterns and financial
constraints.
A 1983 survey of public STD facilities throughout Australia found that almost all clinics were located in major cities, leaving large areas of rural Australia unserviced by public clinics.5 Other deficiencies identified included inadequate opening times, lack of facilities in some clinics for treating both men and women, insufficient staff, inadequate contact tracing, limited use of facilities for teaching purposes, and inadequate maintenance of their role as reference centres for other practitioners and agencies. This survey included all 20 public STD facilities Australia-wide, and concluded that these facilities were "inadequate to meet the needs of the population".5 Over the past decade there have been many changes to sexual health care, including more clinics and better training for staff. Consequently, we felt that it was timely to establish what STD services were available from public and private STD clinics and Family Planning Clinics (FPCs); whether STD clinical services were adequately staffed; to determine the range of diagnostic, treatment, and contact-tracing services; and whether clinics were providing the community with education, counselling and other specialist services. Finally, we compared services in Australia and New Zealand and noted geographic differences in the provision of services within Australia. |
Methods |
In 1993 we identified all STD treatment facilities in Australia and
New Zealand. These included all known public and private sexual
health clinics, and the main Family Planning Clinic (FPC) in each
State. Most facilities were identified through the National
Venereology Council of Australia and the Family Planning
Association of New South Wales, with the remainder found when clinics
identified centres in their area which had been overlooked.
A questionnaire and prepaid addressed envelope were posted to the director (senior doctor or nurse) of each clinic. The 65-item questionnaire sought information about the geographic location and physical structure of clinics, as well as staffing, diagnosis, treatment and contact tracing for the various STDs, specialist medical services, and community and educational programs run by the facility. Non-respondents were sent a second questionnaire two months after the first. |
Statistical analysis | The results were analysed using the Statistical Package for the Social Sciences (SPSS).6 Descriptive statistics, frequency distributions, χ2 and Fisher's exact test were used. There was no weighting of the data by size of clinic. Chi-square tests, when used to compare therapies for the various STDs, involve all treatments, ranging from "most commonly used" to those "never used". When reporting significant differences the χ2 takes into account all these modalities. However, the figures reported show only a description of where the differences lie. The rural/remote areas classification system was used to classify Australian facilities into rural and remote areas by means of postcodes,7 while the New Zealand Yearbook was used to classify those in New Zealand.8 |
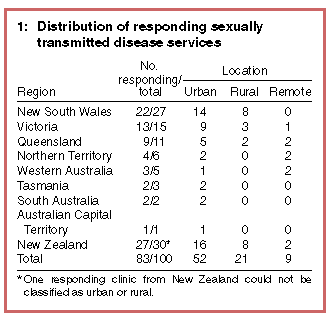
Results | We identified 101 STD facilities, one of which had closed down. From the remaining 100 clinics, 83 questionnaires were returned (72 on the first mailout and an additional 11 on the second). |
Geographic distribution | Fifty-six clinics were in Australia and 27 were in New Zealand. Sixty-three per cent were in inner-city and suburban areas, 26% in rural areas and 11% in remote areas (Box 1). Seventy-nine clinics (95%) were public and four (5%) were private.There were 71 STD clinics and five FPCs. One private facility identified itself as a specialist venereology practice, and three as general practices specialising in STDs and HIV. The remaining three clinics identified themselves as "other". Seventy-nine clinics (95%) were open to both men and women. |
Staffing |
Box 2 shows that the median number of patients seen per year decreased
as the facilities became more remote. Clinics in Australia saw a
greater median number of patients per year and had more doctors,
nurses, laboratory and clerical staff than those in New Zealand.
However, the only significant difference was for full-time
equivalent clerical staff, with a median of 1 (range, 0-4.3) for
Australia, compared with 0 (range, 0-2.4) for New Zealand (P =
0.002).
The most common duties of nurses at STD facilities included history taking, clinical examination and testing for STDs. Only 26% of clinics allowed nurses to prescribe treatment; more nurses in rural and remote areas were allowed to prescribe treatment than those in urban areas (36% v. 16%), but this difference was not significant. Nurses took histories "sometimes" (options were "always", "sometimes" and "never") in over 80% of clinics in New South Wales, compared with 39% in Victoria (P = 0.02), and examined patients at 70% of NSW clinics, compared with 22% in Victoria (P = 0.02). There were no significant differences in nursing duties between New Zealand and NSW. However, comparing New Zealand with Victoria showed that New Zealand nurses were more likely to take histories (P = 0.003), examine patients (P = 0.005), and test patients for STDs (P = 0.002). |
Diagnosis and treatment |
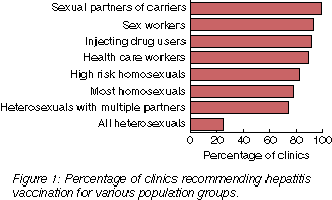
Gonorrhoea: Eighty-one clinics (99%) used culture, and 77 (94%) used Gram stain to diagnose gonorrhoea. The three most common treatments for gonorrhoea were amoxycillin plus probenecid, ciprofloxacin, and ceftriaxone, used in 63%, 24% and 12% of clinics, respectively (Box 3). New Zealand clinics were more likely than Australian clinics to use amoxycillin plus probenecid as first-line therapy for gonorrhoea (93% v. 49%; P = 0.01). Urban clinics in both Australia and New Zealand were more likely to use ciprofloxacin than rural and remote clinics (51% v. 21%; P = 0.02). Syphilis: Forty-seven clinics (57%) used dark-ground microscopy for diagnosing primary syphilis. For screening, 61 (74%) used the rapid plasma reagin (RPR) test, 58 (71%) the Treponema pallidum haemagglutination antibody (TPHA) test, and 25 (31%) the Ven ereal Disease Research Laboratory (VDRL) test. Fifty Australian clinics (91%), compared with 11 New Zealand clinics (41%), used the RPR test for screening (P < 0.001). The fluorescent treponemal antibody test (FTA) was used by 61 (75%) clinics to confirm the diagnosis. Procaine and benzathine penicillins were the first-choice treatments in all facilities. Chlamydia and non-gonococcal urethritis: Chlamydia was most commonly diagnosed by direct immuno fluorescence (DIF), which was used by 44 clinics (54%), followed by: enzyme immunoassay (EIA) from swabs, 39 clinics (48%); culture from swabs, 35 clinics (43%); and EIA from urine, 10 clinics (12%). Australian clinics were more likely to use culture to diagnose chlamydial infection than New Zealand clinics (58% v. 11%; P < 0.001), while New Zealand clinics were more likely to use DIF than Australian clinics (74% v. 44%; P = 0.01). Urban centres were significantly more likely to use culture to diagnose Chlamydia than rural and remote centres (58% v. 17%; P < 0.001). Most clinics used doxycycline as first-line treatment for Chlamydia and non-gonococcal urethritis (92% and 91%, respectively). Genital herpes: Herpes simplex virus (HSV) was diagnosed by viral culture (79 clinics [96%]), EIA (16 clinics [20%]), and serological testing (10 clinics [12%]). Acyclovir was used to treat primary HSV infections by 79 clinics (98%), for long term suppression by 70 clinics (86%), and for recurrences of HSV infection by 58 clinics (72%). Human immunodeficiency virus (HIV): All the clinics surveyed routinely offered counselling and voluntary HIV testing to all persons considered to be at risk of HIV infection. Fifty-two clinics (64%) had facilities to manage HIV- positive patients. Forty-one clinics (51%) provided antiretroviral therapy and prophylaxis and treatment of opportunistic infections. Genital warts: Cryotherapy was the first-line treatment for human papillomavirus infection in 55 clinics (69%), and podophyllin was the second most common treatment option, used in 17 clinics (21%). Australian clinics were more likely to use podophyllin than New Zealand clinics (16/53 [30%] v. 3/27 [11%]; P = 0.03). Trichomoniasis and bacterial vaginosis: To diagnose trichomoniasis, wet-film microscopy was the most common technique, used in 67 clinics (83%), while culture was used by 39 clinics (48%). Gram staining was the most common diagnostic technique for identifying bacterial vaginosis (74 clinics [93%]). Alternative diagnostic techniques used included culture to identify Gardnerella vaginalis and other anaerobes (56 clinics [70%]), vaginal pH testing (44 clinics [55%]), and the potassium hydroxide test (which identifies volatile amines) (41 clinics [51%]). Most clinics used metronidazole to treat trichomoniasis (53/80 [66%]) and bacterial vaginosis (50/79 [63%]). Viral hepatitis: Forty-four clinics (55%) offered antibody testing for hepatitis A and 79 (98%) for hepatitis C. Australian clinics were significantly more likely to offer antibody testing for hepatitis A than New Zealand clinics (36/54 [67%] v. 8/26 [31%]; P = 0.003). When screening for hepatitis B infection, 65 clinics (81%) meas ured surface antigen (HBsAg), 57 (71%) measured core antibody (HBcAb), and 48 (60%) measured surface antibody (HBsAb). Figure 1 shows clinic policies for hepatitis B vaccination. Vaccination was recommended for health care workers by 52/53 (98%) Australian clinics, compared with 19/27 clinics (70%) in New Zealand (P < 0.001); for heterosexuals with multiple partners, these figures were 44/53 (83%) and 15/27 (56%), respectively (P = 0.01); and for intravenous drug users (IVDUs), 52/53 (98%) and 21/27 (78%), respectively (P = 0.002). |
Contact tracing |
Seventy-one clinics (87%) offered contact tracing for patients with
STDs. Many centres employed multiple techniques. In 67 clinics
(94%), staff negotiated with patients to advise their own contacts,
53 clinics (75%) used face-to-face discussions between contacts and
clinic staff, 45 (63%) had staff visit contacts at home, and 38 (54%)
traced contacts by phone.
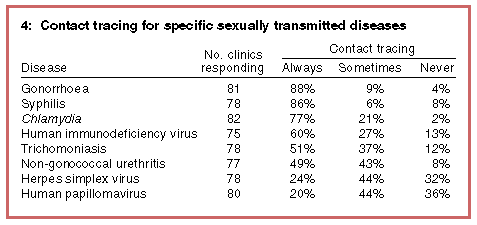
Twenty-one of the 71 clinics (30%) employed specially trained contact tracers. Doctors were involved in contact tracing in 22 clinics (28%), counsellors in 23 (32%), and nurses in 44 (62%). Patients were encouraged to inform their own contacts in 28 clinics (39%). All 21 clinics in NSW stated that they undertook contact tracing, compared with 9/13 (69%) clinics in Victoria (P = 0.01), and NSW clinics were significantly more likely to involve counsellors in contact tracing than Victorian clinics (62% v. 8%; P = 0.002). There were a number of differences in contact-tracing policies between Australia and New Zealand. Australian facilities were more likely than New Zealand clinics to involve doctors (22/55 [40%] v. 1/27 [4%]; P = 0.001), less likely to trace contacts by telephone (21/55 [38%] v. 18/27 [67%]; P = 0.02), and less likely to trace contacts by having staff visit them (22/55 [40%] v. 23/27 [85%]; P < 0.001). Box 4 summarises the contact-tracing practices for each STD. Most clinics always traced contacts of patients with gonorrhoea, syphilis, Chlamydia , HIV and trichomoniasis. Only one of the five FPCs (20%) provided contact tracing, compared with 65 of 66 (98%) sexual health clinics (Fisher's exact test, P < 0.0005). |
Additional services provided by STD clinics |
The facilities surveyed offered a range of additional services.
Eighty-one facilities (98%) offered counselling before and after
HIV testing, 78 (95%) functioned as a referral service for further
specialist counselling, 68 (84%) offered family planning advice,
and 48 (59%) offered relationship counselling. Other services are
outlined in Figure 2.
Sixty-eight (82%) of the responding clinics offered education programs to other health professionals, schools or youth groups, while 46 (55%) offered some form of higher education/university courses. |
Discussion |
Our findings show that the number of STD facilities in Australia
increased considerably over the past decade, and that facilities in
New Zealand and Australia were broadly comparable (although, in
Australia, there was still a marked urban predominance of clinics).
Other improvements were that most clinics were open to men and women,
and offered a wide range of teaching and other special services.
Relative to the 1983 survey of Australian STD services by Bradford and
Philpot,5 we found an
increase in the number of nurses and doctors, while the number of
patients seen annually was similar. However, over the past decade
clinics have become more involved in managing patients with complex
medical problems, including HIV, hepatitis B and C, disseminated
herpes simplex virus infection, and cervical intraepithelial
neoplasia.4,9 Increased availability of services does not necessarily lead to improvement in the sexual health of the community. The best measure of improvement is a reduction in the incidence of sexually transmitted diseases. In Australia, disease patterns have been difficult to in terpret because the collection of epidemiological data for most STDs (with the exception of gonorrhoea) from the States and Territories is uneven.4 None the less, there is considerable evidence to suggest that there has been a dramatic reduction in reported cases of gonorrhoea, syphilis and chlamydial infections since 1981,10-12 with the most marked improvements in the large metropolitan centres. By contrast, the incidence of gonorrhoea, syphilis, Chlamydia and donovanosis remained extremely high in many rural Aboriginal communities, particularly in the Northern Territory, far north Queensland, and the Pilbara and Kimberley regions of Western Australia.13-15 These areas all have a limited number and quality of sexual health facilities, and improvements in clinical services and intervention programs are urgently needed. Our findings show considerable consistency in diagnosis and treatment of STDs throughout Australia and also between Australia and New Zealand, with most facilities following the diagnostic and treatment guidelines produced by the United States Centers for Disease Control (CDC)16 or those of the Venereology Society of Victoria.17 However, we did identify some differences between Australia and New Zealand in the treatment of first choice for gonorrhoea, the method of detecting chlamydial infection, and in the likelihood that hepatitis A tests would be requested. There were also differing policies on hepatitis B vaccination and on tracing contacts of patients with STDs. Varying patterns of antibiotic resistance in strains of the organism that causes gonorrhoea (penicillinase-producing Neisseria gonorrhoeae ) were more common in Australia, particularly along the east coast,18 than in New Zealand, and resistance to quinolones occurs in strains infecting a sizeable minority of travellers from the Philippines and neighbouring countries;19 this may explain the difference in antibiotic treatment. Other factors contributing to the differences in practice include local availability of diagnostic tests, variation in populations attending clinics (travellers,20,21 sex-industry workers22,23 and homosexual men24-26 ) and local variations in clinical practice. The differences in hepatitis A testing policy and hepatitis B vaccination policy are intriguing, and may include differing financial arrangements between the clinical services in the two countries, availability of testing and vaccination from other sources (in particular, the recommendation for universal HBV vaccination in New Zealand),27 and differing populations28,29 and perceptions of risk.30 Differences in contact-tracing policies probably reflect availability of staff, local working practices, or variation in the understanding of contact tracing. There are a few minor differences between urban clinics and those in rural and remote settings, with a greater proportion of urban clinics using ciprofloxacin to treat gonorrhoea, using culture to diagnose Chlamydia , and requesting hepatitis A testing. This also reflects differences in the population groups attending different clinics (urban centres attract a higher proportion of overseas travellers, sex workers and homosexual and bisexual men than rural and remote centres), antibiotic sensitivities and local availability of tests. Overall, the availability of contact-tracing services was excellent. However, it is of some concern that 4% of clinics never and 9% only sometimes traced contacts for gonorrhoea, 8% never traced contacts for syphilis and 2% never traced contacts for Chlamydia . Although we surveyed only five FPCs, our finding that only one of these five provided contact tracing is of concern, particularly with Chlamydia , which is often diagnosed within these facilities.31 Barriers to contact-tracing services within FPCs should be explored and solutions considered. One possible solution would be to encourage closer links between sexual health and family planning services. Our findings in this survey should be of assistance in future sexual health service planning. In addition, the remarkable consistency in the diagnosis, treatment and contact tracing of STDs in Australia and New Zealand should encourage the development of national guidelines. |
Acknowledgements | We thank Professor G Berry and Associate Professor B Donovan for their assistance with this manuscript. |
References |
(Received 9 Apr, accepted 1 Nov, 1996) |
Authors' details
Academic Unit of Sexual Health Medicine, Sydney Hospital, Sydney, NSW.Caron Marks, BSc, MA, Research Assistant;
Robin L Tideman, MB BS, Clinical Research Coordinator;
Adrian Mindel, FRACP, MD, Professor of Sexual Health Medicine.
Reprints: Ms C Marks, Academic Unit in Sexual Health Medicine, Sydney Hospital, GPO Box 1614, Sydney, NSW 2001.
E-mail: cmarks AT extro.ucc.su.oz.au
- - To top of article - ©MJA 1997
<URL: http://www.mja.com.au/>
© 1997 Medical Journal of Australia.
Received 4 March 2026, accepted 4 March 2026
- Caron Marks
- Robin L Tideman
- Adrian Mindel