Hepatitis C transmission on the north coast of New South Wales: explaining the unexplained
Tim J Sladden, Alan R Hickey, Therese M Dunn and John R Beard
MJA 1997; 166: 290
For comment see Wodak
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au/>".
Abstract - Introduction - Methods - Results - Exposures - Transmission to sexual partners and offspring - Discussion - Acknowledgements - References - Authors' details
- - ©MJA1997
Abstract |
Objective: To determine the routes of hepatitis C
virus (HCV) transmission in an Australian community. Design: Questionnaire-based, cross-sectional survey of notified HCV cases. Subjects and setting: All cases notified to the New South Wales North Coast Public Health Unit between 1 January 1993 and 30 September 1994. Outcome measures: Frequency of potential transmission exposures (parenteral and sexual); most likely primary exposure; HCV infection rates in sexual partners and offspring. Results: 467 subjects responded (47% of resident cases). Of these, all but one reported actual or potential blood exposures (injecting drug user [IDU], 85%; IDU with sharing of injection equipment, 76%; pre-1990 blood transfusions, 6%; other blood exposures, 8%). Most subjects reported multiple exposures and none reported sexual contact as the only potential exposure. Of 233 sexual partners tested for HCV, 83 were positive; 54 of these were questioned and all had other parenteral exposures. Only three children out of 91 children tested were positive for HCV (two expressing maternal antibodies). Conclusions: In contrast with previous studies, possible HCV transmission modes were identified for almost all respondents. Most respondents in this community were IDUs. Non-parenteral transmission appeared minimal. Novel approaches to preventing HCV transmission in IDUs are needed. |
Introduction |
Few studies have investigated transmission of hepatitis C virus
(HCV) in representative, population-based samples.1-3 Most previous studies have
examined patient series,4-6
those at risk (e.g., transfusion recipients and injecting drug users
[IDUs])7-9 or other
particular groups (e.g., blood donors, pregnant women and
prisoners).10-12 Previous
studies have also failed to identify exposures in significant
numbers of cases (up to 45% of respondents).1-5,8 HCV transmission is predominantly parenteral13 (via shared drug injection equipment, infected blood products [almost entirely before screening was introduced in February 1990], unsterile skin penetration practices [e.g., tattooing, ear/skin piercing, acupuncture], needlestick and "sharps" injuries and shared personal items, such as toothbrushes and razors14 ). However, patient-to-patient transmission (via contaminated anaesthetic circuitry)15 and surgeon-to-patient transmission (via percutaneous injury)16 have both been demonstrated. Sexual transmission without blood contact appears rare,5,6,8,13 but the risk of blood exposure may be increased by sexual contact during menstruation and anal intercourse. Vertical transmission also appears rare.8,13,17 Both sexual and vertical transmission appear viraemia-dependent,8,9,13,17 and may be facilitated by genital lesions.6,17 Nipple trauma may enable postnatal transmission.18 Household transmission is probably restricted to infected personal items.19 Arthropod vectors have not been identified. Despite evidence that there is no risk of transmission via casual contact, community concern about hepatitis C transmission remains evident in the discrimination reported anecdotally by people with HCV. Clarification of how HCV is transmitted is needed both to allay this concern and to allow the development of new prevention strategies.20 One focus of the current Australian hepatitis C epidemic is the north coast of New South Wales (NSW), where the notification rate (201/100 000 residents) is double the NSW rate (103/100 000 residents),21 and nearly three times the Australian average (74/100 000 residents).22 We investigated the mode of transmission in notified cases in residents on the NSW north coast over a 21-month period. |
Methods |
All people diagnosed with HCV infection (through duplicate second
generation anti-HCV antibody tests) who were notified to the North
Coast Public Health Unit between 1 January 1993 and 30 September 1994
were invited to participate. Subject name, diagnosis and contact
address were verified with attending doctors. Questionnaires were
mailed to subjects with covering letters, consent statements, and
reply-paid return envelopes. Non-respondents were
recontacted by mail six weeks later. Data, with identifying
codes removed, were entered into a restricted-access database.
We developed a questionnaire that included demographic questions, a checklist of possible ways the subject may have contracted hepatitis C (see Box 1) and questions about the HCV status of the subject's current sexual partner and, for women, of their children. The questionnaire was pilot-tested on clients of a local sexual health service. The study and questionnaire were approved by the North Coast Region Health Service Ethics Committee. For subjects with multiple potential exposures, exposures were ranked according to expected risk, and the exposure with the highest risk was considered the most likely primary exposure. Parenteral exposures were assumed to be higher risk than sexual exposure and ranked as:
Transmission between study subjects and their current sexual partners was investigated; a modified questionnaire which included only the checklist of potential transmission routes was mailed to all HCV-positive partners. Household and social contact were examined when no other exposure was reported. Subjects with only low-risk or no parenteral exposures were offered testing for HCV RNA by polymerase chain reaction (PCR) to confirm their hepatitis C status. Demographic characteristics of respondents and non-respondents were compared with chi-squared tests and t tests, and sharing of injection equipment by current and former IDUs was compared with chi-squared tests. |
Results | Of 1487 notified cases, 487 were excluded (395, temporary residents or non-residents; 67, with address unknown; seven, aged under 18 years; 18, other reasons), leaving a study population of 1000. Questionnaires were returned by 467 (46.7%). Respondents differed significantly from non-respondents in sex and age distributions: respondents included a significantly higher proportion of women than non-respondents (respondents: 219/467 [47%]; non-respondents: 212/533 [40%]; chi-squared = 4.42, P = 0.035) and were significantly older (respondents: mean age, 37.4 years; 95% confidence interval [CI], 36.6-38.3; range, 18-86; non-respondents: mean age, 34.9 years; 95% CI, 34.4-35.5; range, 18-62); t [unequal variances] = 4.70; df = 852, P < 0.001). |
Exposures |
Potential HCV exposures are shown in Box 1. Almost all 467 respondents
reported at least one potential transmission exposure and most
reported multiple exposures. The potential exposure with the
highest expected risk was classified as the most likely primary
exposure.
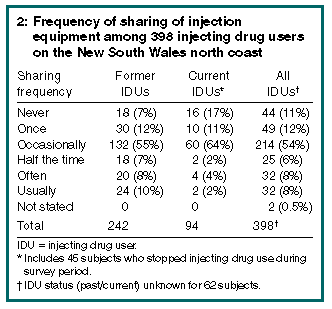
Most respondents (398 [85%]) were IDUs (222 men, 176 women), including 287 (72%) former IDUs (although 45 [11%] of these reported stopping during the survey period). None of the 19 subjects aged over 60 years were IDUs. Of the IDUs, 354 (89%) reported having shared injection equipment. Frequency of sharing injection equipment by IDU status is shown in Box 2 (below). Current IDUs reported sharing significantly less than former IDUs ( chi-squared = 5.82; df = 1; P= 0.016). Hepatitis B infection was reported by 184 subjects (39%); 175 of these (95%) were IDUs.
All but 11 sharers of injection equipment and all non-sharer IDUs reported other parenteral exposures (Box 1). Among subjects who were not IDUs, the most likely primary exposure (Box 1) was pre-1990 blood transfusion for 30 (6%), dialysis for two (0.4%), needle-stick injuries for six (1%) (including three health care workers, one with a known exposure to hepatitis C virus, a garbage collector and the partner of an IDU), tattooing for four (0.9%), blood splashes for four (0.9%), origin in a "high-risk" country for three (0.6%), post-1989 blood transfusion for two (0.4%), skin piercing for 13 (3%) and medical procedures for four (0.8%). About a third of subjects (151 [ 32%]) reported past or current HCV-positive sexual partners. However, 147 (97%) also had potential blood exposures (141, IDU; three, blood transfusion; and one each, tattoos, needlestick injury and blood splash). The remaining four had low-risk potential blood exposures (pierced ears or skin for three and a clinical procedure for one). Thus, sexual contact did not occur without concurrent or potential blood exposure(s). Sharing of personal items (e.g., toothbrushes) also could not be excluded for subjects reporting sexual contact. For 15 subjects, no sexual exposure and only low-risk potential parenteral exposures were reported. Another subject reported no risk factors. Polymerase chain reaction (PCR) was offered to these 16 to confirm their anti-HCV antibody test results. Ten were lost to follow-up (including the subject with no risk factors), three were confirmed HCV-positive (all had had clinical procedures, two had pierced ears and the third had social contact with an HCV-positive person) and three were HCV-negative, indicating either resolved infections or false positive initial antibody results. |
Transmission to |
Three-hundred-and-twenty subjects (69%) had current sexual
partners. Of 233 partners who had been tested for HCV, 83 (36%) were
positive, 138 negative and 12 had unknown results. Of the positive
partners, 80 (96%) were partners of IDUs, with independent
parenteral exposures determined for 54 (68%) and unknown for the
rest. Of the negative partners, 70 (51%) practised "unsafe" sex
(defined as unprotected oral, anal or vaginal sex) with study
subjects.
Among the 219 women subjects, 173 had had children; 56 had had one child tested for HCV and 35, a second child. Only three children were anti-HCV positive -- two infants expressing maternal anti-HCV antibodies, and a three-year old. |
Discussion |
Many studies have investigated HCV transmission in limited patient
series or specific groups,4-12
and full identification of exposures has been difficult.1-5,8 Our study was a
population-based survey, and we were able to identify potential
blood exposures for 99% of respondents. However, the response rate
was low (47%), reflecting the difficulties of community-based
surveys and, possibly, community sensitivities about bloodborne
viruses. In addition, there were small but statistically
significant age and sex differences between respondents and
non-respondents, with a higher proportion of women among
respondents and most older subjects responding. However, none of the
19 subjects aged over 60 years were IDUs. We suggest that non-IDUs
would be more likely to respond than IDUs, and that a higher response
rate in non-IDUs accounted for the age difference between
respondents and non-respondents. Questionnaire comprehension was
not thought to be a problem as the north coast population is
predominantly English-speaking (96%).24 The high proportion of subjects
declaring use of illicit drugs in the 60 years and under age group (89%)
implies they were reporting truthfully. Therefore, we postulate
that the respondents were representative of those infected with HCV
in this community. If more respondents had denied injecting drugs,
selection bias due to non-response of IDU subjects might have been a
legitimate concern.
Our results suggest that, on the NSW north coast, injecting drug use with sharing of injection equipment accounted for transmission in 76% of all people with hepatitis C. Transmission during injecting drug use remained possible in a further 9% who denied sharing injection equipment, as unrecognised contamination of such equipment (e.g., spoons, filters, water or swabs), poor recall or denial of sharing may have occurred. Apart from IDU, other high- or medium-risk blood exposures appeared to be responsible for a further 10% of respondents, and all but one of the remaining 5% had low-risk or potential blood exposures, with 1% of these also having sexual contact. While rates of IDU (past or current) may be relatively high on the NSW north coast, we suggest that almost all HCV transmission is via blood exposure, with varying proportions of different types of blood exposure in different populations. Thus, there appeared to be minimal HCV transmission via sexual, perinatal, household, occupational or social contact, provided blood exposures were avoided. Supplemental testing identified some false positives among the few subjects without obvious blood exposures. While a third of subjects had had sexual contact with HCV-positive partners, all of these had additional blood exposures (high risk for 95%, medium risk for 2% and low risk for 3%). Our results should help allay community concern about HCV transmission via casual contact. Sexual transmission appears to be minimal, but the risk may increase with menstruation, anal sex, concurrent STDs that involve scratching, sores or blisters, and increasing viraemia (often observed in early, acute stages of HCV infection). Uninfected partners should also avoid oro-facial abrasions of infected sexual partners (e.g., from toothbrushing or razor cuts). People with hepatitis C who are contemplating having children would be advised to seek medical advice regarding their HCV-RNA PCR status, hepatic enzyme function, and clinical symptoms as markers of viral activity. The low frequency of unexplained transmission in this study (< 1%) was almost entirely attributable to the high rate of reporting of injecting drug use. Other studies have found much lower rates of injecting drug use in HCV-positive subjects, but have been unable to explain transmission in a much higher proportion (27%-45%),1,3-5 possibly because of reluctance to admit illicit drug use. In our study, the privacy afforded by the self-administered questionnaire may have encouraged more truthful reporting than may occur in an interview or clinic situation. Many IDUs were former users, suggesting not only reluctance of current IDUs to participate, but also that much HCV infection is due to past drug use. Current IDUs shared injection equipment significantly less often than former IDUs, indicating increased awareness of the dangers and the effectiveness of needle and syringe exchange programs. However, sharing of injection equipment remains the commonest route of transmission of HCV, responsible for an estimated 10 000 new infections each year in Australia.25 The proportion of cases due to IDU will increase with blood product screening. This emphasises the need for transmission prevention and harm-minimisation programs, especially targeting adolescents before any experimental drug-taking. Community development and peer education of IDUs to promote safer injecting practices should be strengthened.26 The impacts of improved access to needle and syringe exchange and methadone programs, campaigns to encourage non-injecting routes of drug administration, development of non-reusable syringes,25 supply of heroin to registered users27 and provision of "safe-house" injecting venues on HCV transmission all need to be investigated. |
Acknowledgements | Kieran Mutimer and staff at the Lismore Sexual Health and AIDS Service assisted with piloting and circulating questionnaires. |
References |
|
(Received 8 Feb, accepted 20 Nov, 1996)
Authors' details
North Coast Public Health Unit, NSW Health Department, Lismore, NSW.Tim J Sladden, MSc, MPH, Epidemiologist;
Alan R Hickey, RN, Research Assistant;
Therese M Dunn, BAppSc(Comp), Research Assistant;
John R Beard, MB BS, FAFPHM, Director.
Reprints: Mr T J Sladden, North Coast Public Health Unit, NSW Health Department, PO Box 498, Lismore, NSW 2480.
E-mail: tslad AT doh.health.nsw.gov.au
- - To top of article - ©MJA 1997
<URL: http://www.mja.com.au/>
© 1997 Medical Journal of Australia.
Received 4 March 2026, accepted 4 March 2026
- Tim J Sladden
- Alan R Hickey
- Therese M Dunn
- John R Beard