In February 1996, vivax malaria was diagnosed in a man from a remote community in far north Queensland who had not visited a malarious area for the past 19 years. Microscopy and DNA studies of blood from other residents of the community did not identify a source of infection. It was suspected the infection was transmitted by mosquitoes from a neighbour who had been infected in Papua New Guinea, but whose blood was not available for DNA tests.
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia".
Introduction - Clinical record - Investigations - Discussion - Acknowledgements - References - Authors' details
IntroductionWe report a case of Plasmodium vivax malaria that we conclude was acquired in far north Queensland in early 1996. There has been only one other report of malaria acquired in mainland Australia 1 since the country was declared malaria-free by the World Health Organization in 1981. |
Clinical recordA 31-year-old white male presented to a Cairns general practitioner in early February 1996 with a two-day history of fever, headaches, generalised aches and pains, lethargy and loss of appetite. Two days later he was hospitalised with vomiting and dehydration; he was given intravenous fluids and discharged within 24 hours. He remained unwell and consulted his general practitioner again the following day.A full blood count showed: lymphocytopenia (lymphocytes, 0.71 x 10 9 /L; normal, 1.5-4.0 x 10 9 /L); neutropenia (neutrophils, 1.95 x 10 9 /L; normal, 2.0-7.5 x 10 9 /L); and thrombocytopenia (platelets, 33 x 10 9 /L; normal, 150-450 x 10 9 /L). Haemoglobin concentration was in the normal range. On review of the routine blood film, schizonts of P. vivax were noticed, and malaria was diagnosed six days after onset of symptoms. The patient was treated with standard doses of chloroquine and primaquine, 2 and has remained well since. The patient had never received a blood transfusion nor used intravenous drugs. He had travelled to Papua New Guinea (PNG) 19 years before presentation, and for most of 1995 had worked as a tradesperson in a remote Aboriginal community in far north Queensland. Over the 1995 Christmas-New Year period he spent seven days in Cairns, followed by 12 days south of Cairns, at Mission Beach. For the next 24 days before onset of symptoms, he worked at the community in far north Queensland. |
InvestigationsBecause the average incubation period for P. vivax malaria is 15 days (range, 12-17 days), 3 we investigated the possibility that the patient acquired malaria via a mosquito bite while at the community. It lies about 15¡ south of the Equator, 40 km from the coast of the Gulf of Carpenteria and about 450 km northwest from the closest international airport, at Cairns. The climate early in the year is monsoonal -- hot and humid with intermittent, heavy rainfall. Most of the population of about 1200 is Aboriginal.Investigations of source: After diagnosis of the index case, blood films were collected from 20 residents of the community who were considered possible sources of infection. These comprised:
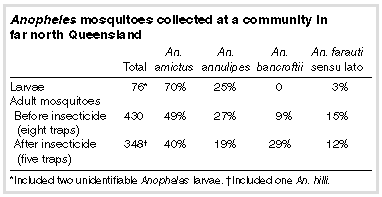
Malaria parasites were seen in the blood films of only one of these 20 people. This person was Melanesian, had arrived in Australia from PNG in November 1995 and had taken no malaria-prophylactic drugs. No parasites were found in thin peripheral blood films, but three early ring trophozoites were seen in thick films. Although definitive species identification could not be made on morphological grounds, DNA amplification detected only P. falciparum (A Baddeley, Centre for Public Health Sciences, Brisbane, and A Saul, Queensland Institute of Medical Research, Brisbane, personal communication). The person was treated accordingly. 2 No evidence of malaria was found by DNA amplification in the other four people tested. The woman whose blood was not available for DNA amplification was also Melanesian, from PNG; she had lived in Australia for about four years and was related to the person with P. falciparum parasitaemia (who was staying with her). Although her house was about 2 km away from the home of the index patient, the latter often spent the evening socialising nearby. Both homes are about 50 m from a creek that runs along the periphery of the community. Investigations of vector: Two days after diagnosis of the index case, surface waters at the community (such as puddles, wheel ruts, ditches, swamps and drains) were surveyed for mosquito larvae with a standard 350-mL dipper. Adult mosquitoes were collected in Centers for Disease Control (CDC) light traps, baited with carbon dioxide and 1-octen-3-ol. Traps were placed beside the creek, in the central built-up part of the community and near the homes of the index patient and of the person with P. falciparum parasitaemia and his relative. Larvae were stored in 70% ethanol and adult mosquitoes at -70oC before identification. 4 Results are shown in the Box. Most Anopheles larvae were collected from small puddles and wheel ruts near the creek. Most adult Anopheles mosquitoes were also collected near the creek, with fewer at the two houses, and very few from the central part of the community. A larvicide, ( S )-methoprene, was used to treat surface waters where Anopheles larvae were breeding, and a residual insecticide, deltamethrin, was used for the thickly vegetated zone along the creek (along the periphery of the community). Further CDC light traps were set a week after initial mosquito collections, in the same locations along the creek as previously. Many Anopheles mosquitoes (12%, An. farauti s.l.) were still present (see Box). |
DiscussionWe believe that this case represents local mosquitoborne transmission of P. vivax malaria at the community in far north Queensland, because:
When an isolated case of malaria cannot be epidemiologically linked with another case of malaria, it is defined as "cryptic". 8 However, for the above reasons, we believe that this was a case of "introduced" malaria (malaria transmitted by mosquitoes from an imported case in an area where malaria does not usually occur). 8 Two episodes of locally acquired malaria in mainland Australia in 15 years attest to the rarity of local transmission, 6 despite frequent importations, particularly into the malaria-receptive north of Australia. 9 Nevertheless, sensitive and timely surveillance must be maintained to ensure that locally acquired cases are promptly recognised and investigated. Intensive mosquito-control measures may be needed, and other cases should be sought and treated promptly. Treatment should include primaquine as a gametocidal agent for P. falciparum . 10 |
AcknowledgementsWe commend Heather Moseley for detecting malaria parasites in the index patient's blood film when malaria was not expected. We wish to thank Dr Bill Glavin and the health staff at the community for assistance with the investigation. |
References
Received 5 Aug, accepted 26 Nov 1996 |
Authors' details
Tropical Public Health Unit, Cairns, QLD.Dianne L Brookes, RN, Public Health Nurse; Scott A Ritchie, PhD, Medical Entomologist; Andrew F van den Hurk, BAppSc, Vector Control Officer.
Cairns Base Hospital, Cairns, QLD.
Julie R Fielding, BAppSc, Supervising Scientist (Haematology); Mark R Loewenthal, DTM&H, FRACP, Infectious Diseases Physician.
No reprints will be available. Correspondence: D L Brookes, RN, Tropical Public Health Unit, PO Box 1103, Cairns, QLD 4870.
E-mail: troppub AT citec.qld.gov.au
- Dianne L Brookes
- Scott A Ritchie
- Julie R Fielding
- Mark R Loewenthal