Passive smoking and respiratory function in very low birthweight children
Lex W Doyle, Geoffrey W Ford, Anthony Olinsky, Annette M L Knoches and Catherine Callanan
For editorial comment see Woodward & Jamrozik
Readers may print a single copy for personal use. No further reproduction or distribution of the articles should proceed without the permission of the publisher. For permission, contact the Australasian Medical Publishing Company
Journalists are welcome to write news stories based on what they read here, but should acknowledge their source as "an article published on the Internet by The Medical Journal of Australia <http://www.mja.com.au/>".
Abstract - Introduction - Methods - Results - Discussion - Acknowledgement - References - Authors' details
- ©MJA1997
Abstract |
Aim: To determine if an adverse relationship exists
between passive smoking and respiratory function in very low
birthweight (VLBW) children at 11 years of age. Setting: The Royal Women's Hospital, Melbourne. Patients: 154 consecutive surviving children of less than 1501 g birthweight born during the 18 months from 1 October 1980. Methods: Respiratory function of 120 of the 154 children (77.9%) at 11 years of age was measured. Exposure to passive smoking was established by history; no children were known to be actively smoking. The relationships between various respiratory function variables and the estimated number of cigarettes smoked by household members per day were analysed by linear regression. Results: Most respiratory function variables reflecting airflow were significantly diminished with increasing exposure to passive smoking. In addition, variables indicative of air-trapping rose significantly with increasing exposure to passive smoking. Conclusions: Passive smoking is associated with adverse respiratory function in surviving VLBW children at 11 years of age. Continued exposure to passive smoking, or active smoking, beyond 11 years may lead to further deterioration in respiratory function in these children. |
Introduction |
Passive smoking is associated with several adverse health outcomes
in children, including higher rates of asthma,1 and infections of the upper2 and lower3 respiratory tract. Further,
respiratory function is reduced with passive smoking in children who
have no lung disease,4 as well
as those with lung diseases such as asthma5 and cystic fibrosis.6 To survive the neonatal period, many very low birthweight (VLBW) children (less than 1500 g at birth) require prolonged periods of assisted ventilation, and some may develop bronchopulmonary dysplasia (BPD) and suffer from ongoing respiratory problems as a consequence. We have previously reported the respiratory health to eight years of age of cohorts of children of birthweight 500-999 g (n = 83), 1000-1500 g (n = 114) and > > 2500 g (n = 51).7 Passive smoking was significantly related to the duration of hospitalisation for respiratory problems up to two years of age for all children in that study, but was not associated with changes in respiratory function at eight years of age. In contrast, in another recent cohort study of respiratory function at seven years of age in children of birthweight less than 2000 g Chan et al.8 reported reduced air-flow rates with maternal smoking, but not with smoking by other household members. Because the effects of passive smoking could increase with increasing duration of exposure, the aim of this study was to determine if an adverse relationship exists between passive smoking and respiratory function at 11 years of age in VLBW children. |
Methods |
We studied 154 consecutive surviving children of less than 1501 g
birthweight born during the 18 months from 1 October 1980 at the Royal
Women's Hospital, Melbourne, the largest of the three
tertiary-level perinatal centres in Victoria. Details of the
survival rate and early neonatal care of this cohort have been
described.9,10
Bronchopulmonary dysplasia (BPD) was diagnosed in children who had
required intermittent positive pressure ventilation in the
neonatal period, who had respiratory distress and were still having
oxygen therapy at 28 days of age, and who had an abnormal chest x-ray
consistent with stage III or IV disease (as defined by Northway et al.11 ) at or after 28 days.
A previous report of the respiratory function of this cohort at eight years of age7 included data for some children with birthweights of less than 1000 g born before 1 October 1980. We did not have the resources to measure respiratory function at 11 years of age of the children born before October 1980. Respiratory health was determined by history and examination, and measurement of respiratory function. Children who had required bronchodilators within the previous year for attacks of wheezing were considered to have asthma. Data on passive smoking were obtained by asking the parents about the daily consumption of cigarettes by members of the household. We did not distinguish between mothers and other smokers, or between smoking inside or outside the home. Some data on maternal smoking in pregnancy had been collected in the perinatal period, but were obtained for only one-third of mothers. Children were questioned about active smoking in their parents' absence. As some children had changed households in their lifetimes, we considered those who had lived in any household with smokers over the 11-year period to have been passively smoking during childhood. Two categories of social class were determined -- unskilled or unemployed, and other (professional, skilled or semi-skilled) -- based on the occupation of the family breadwinner. Respiratory function was measured in the Department of Thoracic Medicine at the Royal Children's Hospital, Melbourne, as described previously,7 by personnel blinded to the exposure of individual children to passive smoking. Maximum expiratory flow rates were recorded with a pneumotachograph (Fleisch No. 3, Switzerland) and plotted against volume by integrating flow on an X-Y recorder to obtain flow-volume loops. Maximum flow rates at 75% (VEmax75%), 50% (VEmax50%) and 25% (VEmax25%) of forced vital capacity (FVC), and forced expiratory flow between 25% and 75% of FVC (FEF25%-75%), were measured from the loops. Flow rates were corrected for body size by dividing by vital capacity (VC). Vital capacity, FVC and forced expiratory volume in one second (FEV1) were measured with a water-filled spirometer (Godart Expirograph, Bilthoven, Netherlands) in accordance with standard guidelines, and results at body temperature and pressure saturated with water vapour were expressed as a percentage of the predicted value for age, height and sex.12 Total lung capacity (TLC) and residual volume (RV) were measured in a body plethysmograph (Jaeger Bodyscreen 2, Wurzburg, Germany). Children were not subjected to bronchial provocation tests as these are poorly tolerated, and we were eager to maintain a high degree of cooperation with these and with future respiratory function tests. Not all children could complete all respiratory function tests, either because of poor cooperation, or unavailability or malfunction of equipment on the day of testing. Data were edited and analysed using SPSS.13 Dichotomous variables were contrasted by chi-squared analysis, and continuous variables by t test, or Mann-Whitney U test if the data were skewed. The dose-response relationship between the estimated daily number of cigarettes consumed by members of the household and various respiratory function variables was established by linear regression; linear and quadratic relationships were tested. Data were then analysed by linear regression to adjust for the potentially confounding variables of birthweight, gestational age, birthweight ratio (child's birthweight divided by median birthweight for gestational age14 ), sex, BPD, and asthma; all variables were entered simultaneously, even if they were not statistically significant. Durations of intermittent positive pressure ventilation and oxygen therapy were not included as they were strongly related to BPD. For all analyses, P values of less than 0.05 for any test were regarded as statistically significant. |
Results |
We measured the respiratory function of 120 of the 154 (77.9%)
children at 11 years of age. Of the 34 children not tested, 15 lived in
another State, four lived in another country, 10 refused the tests,
three were lost to follow-up, and two were too disabled to complete the
tests. There were no substantial differences in perinatal variables
between children who did and did not have respiratory function tests
at 11 years of age.
Eighty of the 120 children (66.7%) had been exposed to passive smoking in the household. The only substantial differences in perinatal or subsequent variables between children who were and were not exposed to passive smoking were a significantly longer duration of oxygen therapy and a lower proportion of unskilled or unemployed families in the group not exposed (Table 1). For children exposed to passive smoking, the median number of cigarettes consumed in the household per day was 21 (interquartile range, 15-25). Of the 15 children tested who had developed BPD in the newborn period, three (20%) had asthma at 11 years of age; this proportion was similar for children with asthma at 11 who did not have BPD (22 of 105; 21%).
|
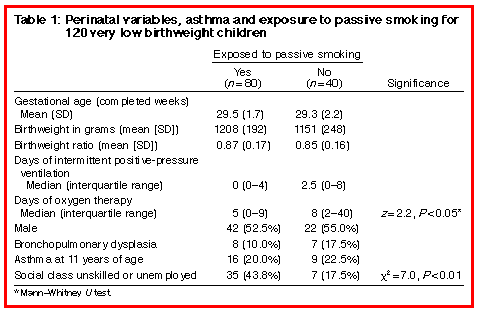 | |
For variables expressed as a percentage of predicted values (FEV1, FVC, RV, TLC), the means of the measured values were all close to their expected values of 100% (Table 2). For all respiratory function variables significantly associated with the dose of passive smoking, a quadratic relationship was more significant than a linear relationship (Table 2, Figures 1 and 2). Most respiratory function variables reflecting airflow (VEmax75%/VC, VEmax50%/VC, FEF25%-75%/VC, FEV1 and FEV1/FVC) were significantly diminished by increasing exposure to passive smoking (Table 2 [below], Figures 1a and 1b). In addition, RV, TLC and RV/TLC rose significantly (consistent with progressive air trapping) with increasing exposure to passive smoking (Table 2 [below], Figure 1c).
| |
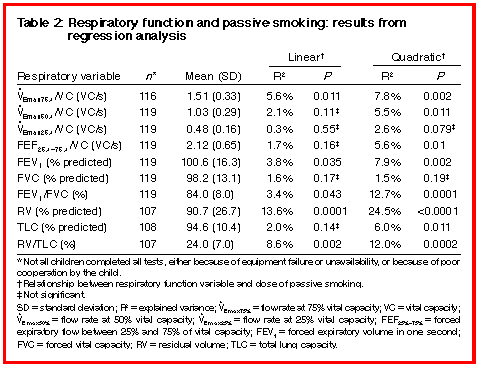 | |
One child was exposed to 115 cigarettes per day, and the next highest exposure was only 70 cigarettes per day. When the child exposed to 115 cigarettes per day was excluded, most of the statistically significant relationships disappeared, except for the increases in RV and RV/TLC (Figure 2). From the multiple linear regression analyses, some variables reflecting flow (VEmax75%/VC, VEmax50%/VC, VEmax25%/VC, FEF25%-75%/VC and FEV1/FVC) were significantly higher in girls. BPD was significantly associated with reductions in some variables reflecting air-flow (VEmax50%/VC, FEF25%-75%/VC, FEV1 and FEV1/FVC), as was asthma (with significant reductions in FEF25%-75%/VC, FEV1 and FEV1/FVC). FVC was significantly lower and VEmax25%/VC significantly higher with lower social class. Birthweight ratio, birthweight and gestational age were not significantly associated with any lung function variable. None of the statistical conclusions relating respiratory function variables with passive smoking were altered by adjusting for all potentially confounding variables, except that the reduction in VEmax50%/VC was no longer statistically significant. | |
Discussion |
Passive smoking was associated with reduced airflow and
air-trapping in VLBW children at 11 years of age, which is consistent
with observations in non-preterm children free of lung disease.4 However, this finding was
different from our observations of these children at eight years of
age,7 when passive smoking
was unassociated with any lung function variable. Chan et al.8 reported reduced flow rates with
smoking by mothers in children of less than 2000 g birthweight at seven
years of age, but they did not measure variables reflecting
air-trapping. We did not distinguish between mothers and other
smokers in the household.
The association between passive smoking and adverse respiratory function in our VLBW children at 11, but not at eight, years of age suggests that the harmful effects of passive smoking take time to become obvious in VLBW children. Moreover, the adverse response seems to accelerate with increasing dose of passive smoking (Figures 1 and 2). We are concerned that continued exposure to passive smoking, or, even worse, active smoking, beyond 11 years will lead to not only further, but also to an accelerating rate of, deterioration in respiratory function. Our results should not be overinterpreted. They were not substantially altered by adjusting for potentially confounding perinatal or other variables, but we did not have data on a wide range of confounding variables. Moreover, they were heavily influenced by one child who lived in a household whose members consumed 115 cigarettes per day. Excluding this child from the analysis, the only remaining statistically significant associations indicated air-trapping with increasing exposure to passive smoking. However, we consider that this child's data should not be excluded just on the basis of heavier-than-average exposure to passive smoking. To remove any doubt about the association between passive smoking and adverse lung function in VLBW children, lung function could be measured in another cohort of VLBW children, or the same cohort when they are older. Parents of VLBW children, particularly those of children who have received assisted ventilation, frequently ask about long-term lung problems. Many variables, such as family history or duration of assisted ventilation and oxygen therapy, may be related to long-term lung problems, but most cannot be altered by the parents. Exposure to passive smoking is one variable associated with poorer respiratory function in VLBW children they can influence. Until there is evidence to the contrary, families of VLBW children should be encouraged to stop exposing their children to cigarette smoke in the household. As there appears to be a dose-response relationship, those who cannot stop smoking should at least reduce their children's exposure to passive smoking. |
Acknowledgement | This study was supported in part by a grant from the Royal Women's Hospital-3AW Community Services Trust. |
References |
(Received 8 Jun, accepted 18 Nov 1995) |
Authors' details
Division of Paediatrics, the Royal Women's Hospital, Melbourne, VIC.Lex W Doyle, MD, FRACP, Paediatrician; and Associate Professor, Departments of Obstetrics and Gynaecology, and Paediatrics, the University of Melbourne.
Geoffrey W Ford, MB BS, FRACP, Paediatrician.
Annette M L Knoches, MB BS, FRCP(C), Paediatrician.
Catherine Callanan, RN, Research Nurse.
Department of Thoracic Medicine, the Royal Children's Hospital,
Melbourne, VIC.
Anthony Olinsky, FRACP, Respiratory Physician.
No reprints will be available. Correspondence: Associate Professor L W Doyle, Department of Obstetrics and Gynaecology, University of Melbourne, Parkville, VIC 3052.
©MJA 1997
<URL: http://www.mja.com.au/> © 1997 Medical Journal of Australia.
Received 4 March 2026, accepted 4 March 2026
- Lex W Doyle
- Geoffery W Ford
- Anthony Olinsky
- Catherine Callanan




