Consumption of oily fish and childhood asthma risk
Linda Hodge, Cheryl M Salome, Jennifer K Peat, Michelle M Haby, Wei Xuan and Ann J Woolcock
For editorial comment, see Thien et al.
Abstract - Authors' details - Introduction - Methods - Results - Discussion - Appendix - Acknowledgements - References - Box 1 - Box 2 - Box 3 - Figure - © MJA 1996 -
Abstract
Objective: To investigate the association between diet and airway disease in children in the light of epidemiological studies suggesting that consumption of fish more than once a week reduces the risk of developing airway hyperresponsiveness (AHR).Design: Diet was assessed by a detailed food frequency questionnaire and airway disease by respiratory symptoms or airway responsiveness to exercise.
Methods: A questionnaire, containing questions about the frequency of eating more than 200 foods, was sent to the parents of 574 children in whom we had measured recent wheeze (by questionnaire), AHR (by exercise) and atopy (by skin prick tests) six months before this study. We defined current asthma as the presence of both recent wheeze and AHR.
Results: Response rate to the questionnaire was 81.5% (n = 468). After adjusting for confounders such as sex, ethnicity, country of birth, atopy, respiratory infection in the first two years of life and a parental history of asthma or smoking, children who ate fresh, oily fish (> 2% fat) had a significantly reduced risk of current asthma (odds ratio, 0.26; 95% confidence interval, 0.09-0.72; P < 0.01). No other food groups or nutrients were significantly associated with either an increased or reduced risk of current asthma.
Conclusion: These data suggest that consumption of oily fish may protect against asthma in childhood.
MJA 1996; 164: 137-140
Introduction
The substantial increase in the prevalence of childhood asthma in the past 20 years has affected both rural and urban communities of westernised countries,1,2 suggesting that local environmental factors, such as exposure to allergens or industrial air pollutants, are not the cause. However, the widespread changes in diet may be responsible.Seaton et al.3 have postulated that increases in the prevalence of asthma may be due to a reduced intake of antioxidant vitamins (beta-carotene, vitamins A, C and E) and mineral cofactors essential for antioxidant defence mechanisms (selenium, zinc and copper) as a result of reduced consumption of meat, fresh fish, fruit and vegetables in Western diets. Reduced consumption of magnesium4 and increased consumption of salt5 have been implicated as risk factors for airway hyperresponsiveness (AHR).
Our own epidemiological studies of Australian schoolchildren have shown that children who eat fish more than once a week have a third the risk of AHR of children who do not eat fish regularly.6 However, these studies did not include other dietary questions, so that fish consumption may have been a marker for another dietary characteristic. Here, we investigate the association between diet, as assessed by a detailed dietary questionnaire, and airway disease, assessed by respiratory symptoms or airway responsiveness to exercise.
Methods
Subjects
In June 1993, a cross-section of 808 children aged 8-11 years from schools randomly selected from all schools within a 10 km radius of Sydney General Post Office had airway responsiveness to exercise, respiratory symptoms and atopy measured and frequency of fish consumption assessed.Ethical approval for the study was obtained from the Ethics Review Committee of the University of Sydney. Permission to approach schools was obtained from the New South Wales Department of School Education and the Catholic Education Office.
Respiratory questionnaire
In June 1993 the parents or guardians of the children completed a standard respiratory questionnaire, with questions on age, sex, ethnicity, country of birth, history of asthma or wheeze in the last 12 months, medication use, and also parents' occupations, history of asthma and smoking. The questionnaire included the question used in previous studies about the dietary consumption of fish - "How often does your child eat a meal that contains fish?" - with the options of replying "never or rarely", "once a week", or "more than once a week".Dietary questionnaire
In October 1993, a food frequency questionnaire (adapted from that developed and validated by the Commonwealth Scientific and Industrial Research Organisation [CSIRO], Division of Human Nutrition, South Australia8,9) was distributed to the selected children, whose parents were asked to complete this for their child's usual eating habits over the last year. The questionnaire identified consumption patterns (daily, weekly, monthly, rarely or never) of more than two hundred foods commonly consumed in Australia. Additional questions on the type of fresh fish consumed and regular consumption of vitamin, mineral or herbal supplements were included. Estimates of sodium intake included naturally occurring sodium in foods, salt added in cooking, at the table and from processed foods.If questionnaires were not returned after one month the parents were contacted by telephone and offers were made to replace the questionnaires, or to provide assistance. In 11 cases, where neither parent spoke fluent English, an interpreter was commissioned to complete the questionnaire with the parents over the telephone.
Returned dietary questionnaires were checked for missing or obviously erroneous information. Parents were contacted by telephone to complete omitted sections or to clarify erroneous information.
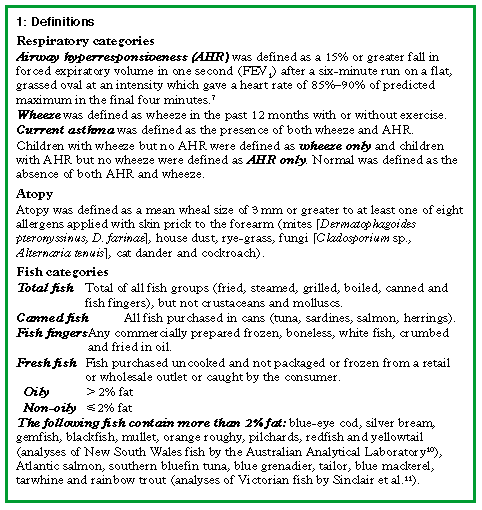
Each food in the dietary questionnaire was allocated to one of 23 different food groups (see Appendix). Diets were analysed for energy, fibre and 39 nutrients (see Appendix). Definitions of respiratory categories, atopy, and categories of fish, plus a list of fish with more than 2% fat, are given in Box 1.
Statistical analyses
The questionnaires were analysed by the Division of Human Nutrition, CSIRO, South Australia, using Australian tables of nutrient composition12 for energy, protein, fat, carbohydrates, vitamins and minerals. The total quantity of food in each food group for every child was converted to a common base of weekly serves with Clinical Reporting Systems software.13 Data were analysed with the statistical package SAS.14The association between fish, food or nutrient intake and respiratory category was analysed categorically using chi-squared tests, and continuously using Student's t tests and analysis of variance.
Some values obtained from the nutrient analysis were well outside what could reasonably be expected in children of this age group. These outliers were excluded from the statistical analysis. The number of exclusions never exceeded nine subjects in any analysis and were not significantly associated with any of the respiratory groups. Logistic regression was used to adjust estimates for the effects of known confounders for the effect of fish consumption on AHR and symptoms of asthma (e.g., sex, race, country of birth, atopy, early respiratory infection, parental smoking and parental asthma). Only those confounding factors found to be significant or approaching significance (P < 0.1) (atopy, parental asthma, early respiratory infection, country of birth) were included in the model.
Results
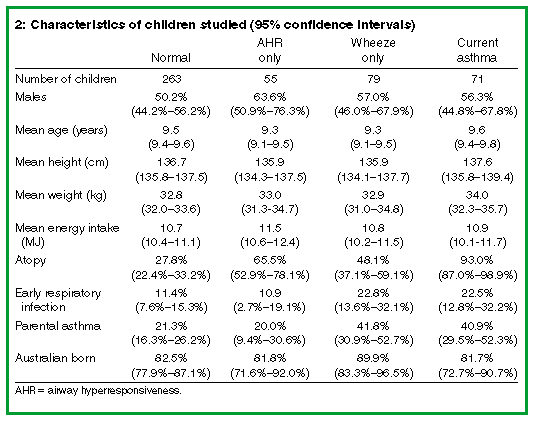
Of the 584 children selected 574 received the dietary questionnaire and 468 completed questionnaires were returned (81.5%). Non-responders were not significantly different from responders in the prevalence of AHR (26.0% v. 27.1%) or fish consumption (46.2% v. 52.1%). Box 2 shows details of the children studied.Children with current asthma did not differ significantly from children with normal airways in the consumption of any nutrient or food group. (Tables showing mean weekly intake in standard serves of food groups and mean daily intake [SD] of nutrients for children with normal airways and children with current asthma are available from the authors.) Children with wheeze only had a significantly higher intake of red meat (P < 0.05), offal meat (P < 0.001) and vitamin B12 (P < 0.03) and a significantly lower intake of mixed vegetables (P < 0.05) than children with normal airways. Children with AHR only consumed significantly more offal meat (P = 0.001) and high fat/high sugar foods (P < 0.001) than children with normal airways. They also had higher intakes of nitrogen, protein, total sugar, cholesterol, potassium nicotinamide, total nicotinamide, calcium, copper, zinc, vitamin B12 (P < 0.05) and refined sugar (P < 0.01).
Total fish intake per week did not differ significantly between children with normal airways (1.2 serves per week; 95% confidence interval [CI], 1.0-1.3), AHR only (1.2 serves; CI, 0.9-1.5), wheeze only (1.2 serves; CI, 0.8-1.5) and current asthma (1.0 serve; CI, 0.8-1.2).
Fresh fish was eaten by 84% (CI, 79.6%-88.4%) of children with normal airways, and by 72% (CI, 61.6%-82.4%) of children with current asthma. When fresh fish was divided into oily and non-oily types, significantly fewer children with current asthma (15.5%; CI, 7.1%-23.9%) included oily fish in their diet than did children with normal airways (30.8%; CI, 25.2%-36.4%; P < 0.05). There were no significant differences in the proportions of children with current asthma (56.3%; CI, 44.8%-67.8%) and normal children (52.9%; CI, 46.9%-58.9%) who ate exclusively non-oily fish. Neither fresh fish consumption nor respiratory disease was significantly associated with socioeconomic status, as defined by the father's occupation, or with the consumption of vitamin, mineral or other dietary supplements (including fish oil).
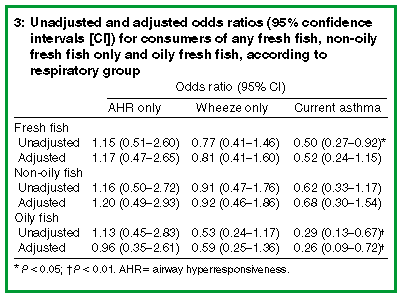
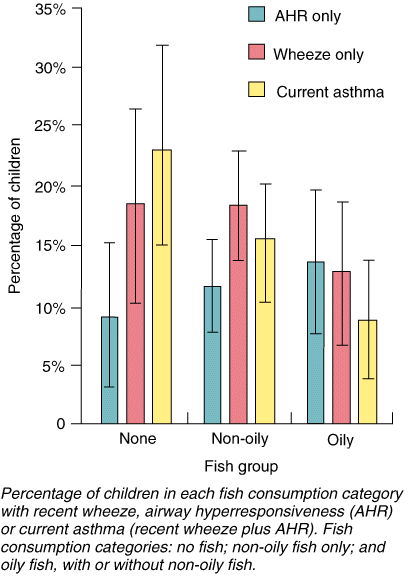
The unadjusted risk (odds ratio) for children having current asthma was significantly lower in those who consumed any fresh fish or oily fresh fish (Box 3). Current asthma was found in only 8.8% (CI, 3.8%-13.8%) of children who ate oily fish, but in 15.6% (CI, 11.2%-20.0%) of those who ate non-oily fish only and 23% (CI, 14.2%-31.8%) of those who never ate fresh fish (Figure). When the results were adjusted for the effects of other known risk factors such as atopy, parental asthma, parental smoking, ethnicity, country of birth, early respiratory illness and sex, only children who ate oily fresh fish had a significantly reduced risk of current asthma. In these children, the risk was almost a quarter that of children who did not eat oily fish (odds ratio, 0.26; CI, 0.09-0.72) (Box 3). Consumption of any fresh fish, whether or not it was separated into oily fresh fish and non-oily fresh fish, did not significantly reduce the risk of AHR only or wheeze only either before or after adjustment for other risk factors.
Discussion
Our study shows that regular consumption of fresh, oily fish is associated with a reduced risk of current asthma. This reduced risk remained significant after adjustment for other known risk factors for asthma, including sex, atopy, parental asthma, parental smoking, early respiratory infection, ethnicity and place of birth.The subjects were selected from a random cross-sectional sample of children which was stratified (on the basis of recent respiratory symptoms and AHR to exercise) to increase the proportion of cases in the study group. The response rate was high (81.5%) and non-responders were not different from responders with respect to AHR or fish consumption. Socioeconomic status was not a confounder for either respiratory illness or fish consumption.
Current asthma was defined as recent wheeze plus AHR to exercise. We have shown previously that current asthma, defined as recent wheeze plus AHR to histamine, identifies a group with severe, ongoing respiratory impairment, while those with AHR only and wheeze only have a milder condition which differs only slightly from the normal group.15
The diets of children with current asthma differed from those of the normal group only in the consumption of fresh, oily fish. In our previous study, more than one serve of fish per week was associated with a reduced risk of asthma,6 but in that study it was not possible to distinguish the effects of oily and non-oily fish. In the study reported here we were unable to detect differences in total fish consumption, possibly because of the smaller sample size. There were no significant differences between respiratory groups in the consumption of non-oily fish, suggesting that parents had not selectively withheld fish from the diets of asthmatic children. It is unclear why consumption of canned and processed fish was not associated with reduced risk of asthma. Processing may alter the integrity or activity of the fatty acids in fish oils.
Several foods and nutrients in the diets of children with AHR only and wheeze only differed significantly from those of the normal group. However, none of these factors differed between the asthmatic and normal groups, suggesting that they are unlikely to have an aetiological role. Intake of offal meats was higher in both the AHR-only and wheeze-only groups, but, as offal meats are eaten by very few children, this may be a type I error. Vitamin B12 intake was also higher in both the AHR-only and wheeze-only groups, but the mechanism by which this could affect respiratory symptoms or AHR is unclear. There were no significant differences between any of the respiratory groups in consumption of sodium, vitamin C, vitamin E, selenium or magnesium. These findings do not support previous evidence that these dietary factors are important in the aetiology of asthma.4,5,16
Reduced risk of current asthma was associated with the consumption of oily fish, but not with non-oily fish. Fish oil contains the omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), which have anti-inflammatory effects.17 Theoretically, EPA could either prevent the development of asthma or reduce its severity by altering two of the cardinal features of asthma, namely airway inflammation and AHR. Supplementation with EPA reduces production of leukotriene B4,18 a chemical mediator responsible for the recruitment of inflammatory cells, such as neutrophils, into the airways. It also reduces production of the cytokine tumour necrosis factor (TNF),19 which increases airway responsiveness.
Fish oil supplements given over 6-10 weeks cause a substantial uptake of EPA in neutrophil membrane phospholipids.18,21 In asthmatics, this may reduce the allergen-induced late asthmatic response,22 but does not change severity of asthma.
In conclusion, we have shown that consumption of oily fish is associated with a reduced risk of asthma in childhood. Although further studies are required to confirm these benefits, public health interventions to increase the consumption of oily fish may reduce the morbidity and prevalence of asthma in children.
Appendix
The 23 food groups were: cereals; dairy products; eggs; red, white, preserved meat and offal; seafood, fried, steamed, canned fish and fish fingers; red, green, white, mixed and other vegetables; legumes; high and low vitamin C fruit and other fruit; high sugar or fat content; and other. The 39 nutrients were: nitrogen; protein; starch; refined, natural and total sugar; total carbohydrate; saturated, monounsaturated, polyunsaturated and total fat; cholesterol; carotene; retinol; vitamin A; thiamine; riboflavin; potassium nicotinic acid and total nicotinic acid; niacin; vitamins B6 and B12; pantothenic acid; biotin; free and total folate; vitamins C, D, E; calcium; copper; iron; magnesium; manganese; phosphorus; potassium; selenium; sodium; and zinc.Acknowledgements
This study was supported by the Fisheries Research and Development Corporation, Australia. The authors thank Dr Katrine Baghurst for allowing us to use the dietary questionnaire, Sally Record and Kay Pender for their help with the nutritional analyses, Elena Belooussova for data organisation and Suzanne Gray for her assistance with collecting the questionnaires. We are grateful for the support of the New South Wales Department of School Education, the Catholic Education Office and the Principals and teachers of all the schools involved. We are especially grateful to the parents and the children who participated in the survey.(©MJA 1996; 164: 137-140)
References
- Robertson CF, Bishop J, Sennhauser FH, Mallol J. International comparison of asthma prevalence in children: Australia, Switzerland, Chile. Pediatr Pulmonol 1993; 16: 219-226.
- Burney P, Chinn S, Rona RJ. Has the prevalence of asthma increased in children? Evidence from the national study of health and growth 1973-86. BMJ 1990; 300: 1306-1310.
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population? Thorax 1994; 49: 171-174.
- Britton J, Pavord I, Richards K, et al. Dietary magnesium, lung function, wheezing, and airway hyperreactivity in a random adult population sample. Lancet 1994; 344: 357-362.
- Burney PG, Neild JE, Twort CHC, et al. Effect of changing dietary sodium on the airway response to histamine. Thorax 1989; 44: 36-41.
- Peat JK, Salome CM, Woolcock AJ. Factors associated with bronchial hyperresponsiveness in Australian adults and children. Eur Respir J 1992; 5: 921-929.
- Haby MM, Peat JK, Mellis CM, et al. An exercise challenge for epidemiological studies of childhood asthma: validity and repeatability. Eur Respir J 1995; 8: 729-736.
- Baghurst KI, Record SJ. Intake and sources in selected Australian subpopulations of dietary constituents implicated in the etiology of chronic diseases. J Food Nutr 1983; 40: 1-15.
- Rohan TE, Record SJ, Cook MG. Repeatability of estimates of nutrient and energy intake: the quantitative food frequency approach. Nutr Res 1987; 7: 125-137.
- Analyses of NSW fish and shellfish. Sydney: Australian Government Analytical Laboratory, 1989.
- Sinclair A, Dunstan GA, Naughton JM, et al. The lipid content and fatty acid composition of commercial marine and freshwater fish and molluscs from temperate Australian waters. Aust J Nutr Diet 1992; 49: 77-83.
- English R, Lewis J. Composition of foods Australia. 1st ed. Vols 1-5. Canberra: AGPS, 1989-1990.
- Clinical Reporting Systems [computer program], version 3.0. Sydney: Clinical Reporting Systems Pty Ltd. 1992.
- SAS [computer program], version 5. Cary, NC: SAS Institute, 1984.
- Toelle BG, Peat JK, Salome CM, et al. Toward a definition of asthma for epidemiology. Am Rev Respir Dis 1992; 146: 633-637.
- Stone J, Hinks LJ, Beasley R, et al. Reduced selenium status of patients with asthma. Clin Sci 1989; 77: 495-500.
- Kremer JM, Jubiz W, Michalek A, et al. Fish-oil fatty acid supplementation in active rheumatoid arthritis. Ann Intern Med 1987; 106: 497-503.
- Lee TH, Hoover RL, Williams JD, et al. Effect of dietary enrichment with eicosapentaenoic and docosahexaenoic acids on in vitro neutrophil and monocyte leukotriene generation and neutrophil function. N Engl J Med 1985; 312: 1217-1224.
- Endres S, Ghorbani R, Kelley VE, et al. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med 1989; 320: 265-271.
- Thomas PS, Yates DH, Barnes PJ. Tumor necrosis factor-alpha increases airway responsiveness and sputum neutrophilia in normal human subjects. Am J Respir Crit Care Med 1995; 152: 76-80.
- Arm JP, Horton CE, Mencia-Huerta J-M, et al. Effect of dietary supplementation with fish oil lipids on mild asthma. Thorax 1988; 43: 84-92.
- Arm JP, Horton CE, Spur BW, et al. The effects of dietary supplementation with fish oil lipids on the airways response to inhaled allergen in bronchial asthma. Am Rev Respir Dis 1989; 139: 1395-1400.
- Dry J, Vincent D. Effects of fish oil diet on asthma: results of a 1-year double blind study. Int Arch Appl Immunol 1991; 95: 156-157.
(Received 31 May, accepted 28 Nov 1995)
Authors' details
Institute of Respiratory Medicine, Royal Prince Alfred Hospital, Sydney, NSW.Linda Hodge, MSc(Med), GradDipNutr&Diet, Dietitian.
Department of Medicine, University of Sydney, Sydney, NSW.
Cheryl M Salome, BSc, Senior Research Officer. Jennifer K Peat, PhD, Senior Research Officer.
Michelle M Haby, MSc, Research Assistant. Wei Xuan, MSc, MApplStat, Statistician.
Ann J Woolcock, MD, FRACP, Professor in Respiratory Medicine.
Reprints: Professor A J Woolcock, Institute of Respiratory Medicine, Royal Prince Alfred Hospital, Camperdown, NSW 2050.
(©MJA 1996; 164: 137-140)
Received 4 March 2026, accepted 4 March 2026
- Linda Hodge
- Cheryl M Salome
- Jennifer K Peat
- Michelle M Haby
- Wei Xuan
- Ann J Woolcock




