The known The identification, management and prognosis of relatively uncommon monogenic diabetes types differ from those of other diabetes forms in young patients.
The new The prevalence of monogenic diabetes identified by clinical risk prediction and genotyping, especially maturity-onset diabetes of the young, was relatively low in a community-based sample of Australians diagnosed with diabetes, but was higher among patients from European than those from non-European ethnic backgrounds.
The implications Clinicians providing care to young Australians with diabetes should be aware of the features of monogenic diabetes, as well as the availability of clinical and genetic tools for confirming the diagnosis.
Several types of diabetes that usually develop relatively early in life are caused by single gene mutations. The two main categories of monogenic diabetes are maturity-onset diabetes of the young (MODY) and permanent neonatal diabetes.1 There are a number of MODY subtypes, most caused by mutations in the glucokinase gene (GCK) or in genes encoding hepatic nuclear factors (HNF genes). Estimates of the overall prevalence of MODY in different populations vary, but MODY probably encompasses 0.3–2.4% of all cases of diagnosed diabetes.2-4 Neonatal diabetes, evident within the first 6 months of life and caused by mutations in genes for proteins involved in insulin secretion, is comparatively rare.5 Timely and accurate diagnosis of MODY and neonatal diabetes is important, as their management and prognosis can differ markedly from those for types 1 and 2 diabetes.
Genetic testing for monogenic diabetes is expensive and available in Australia in only a few specialised laboratories. Various algorithms have been developed that aim to predict the likelihood of monogenic diabetes on the basis of clinical data, and to thereby improve the cost-effectiveness of confirmatory genotyping.6 In the United Kingdom, clinical prediction models have been developed that discriminate between MODY and types 1 and 2 diabetes in patients diagnosed before the age of 35 years.7 However, a potential limitation is that these models have been validated only for patients from a European ethnic background; whether they can be reliably applied in a multi-ethnic setting, such as Australia, is unknown.
We investigated the prevalence of MODY and permanent neonatal diabetes in patients with European or non-European ethnic backgrounds participating in the community-based Fremantle Diabetes Study Phase II (FDS2).8 The results provide an estimate of the frequency of monogenic diabetes in Australia, as well as an assessment of the clinical utility of the UK MODY calculator for patients from a non-European ethnic background.
Methods
Patients
The FDS2 is a longitudinal observational study conducted in a postcode-defined community of 157 000 people residing in and around the city of Fremantle.8 Socio-economic data on income, employment, housing, transportation, and a range of other variables yield an average Index of Relative Socio-economic Advantage and Disadvantage9 of 1033 (range by postcode, 977–1113), close to the national mean (set at 1000; standard deviation [SD], 100). Individuals residing in the catchment area with a clinician-verified diagnosis of diabetes (excluding gestational diabetes) were identified by a variety of means, including searching public hospital databases, notifications by general practitioners, specialists and allied health services, advertisements in local media and pharmacies, and word of mouth. Of 4639 people with known diabetes identified during 2008–2011, 1668 (36%) provided informed consent to participation in FDS2.
The mean baseline age of the participants was 62.0 years (SD, 13.8 years), compared with 61.3 years (SD, 17.4 years) for patients who were identified but not recruited. The proportions of men in the two groups were 52.2% and 52.4% respectively; 90.1% and 89.5% had been diagnosed with type 2 diabetes. None of these differences were statistically significant.
Study procedures
All FDS2 patients undergo face-to-face assessments at entry and then every two years; in the non-interview years patients are asked to complete postal questionnaires.8 Face-to-face assessments include a standardised comprehensive questionnaire, physical examination, and fasting biochemical tests by a single, nationally accredited laboratory. Type of diabetes (including previous diagnosis by a clinician of MODY or neonatal diabetes) was ascertained at entry according to diabetes treatment history (especially insulin use and its initiation relative to diagnosis), body mass index (BMI), age at diagnosis, nature of first presentation, and self-identification. Case records were consulted for evidence of ketonaemia, as well as for data on auto-antibody, serum insulin, and C-peptide levels, and on genotyping. Diabetes was classified as type 1, type 2, latent autoimmune diabetes of adults (LADA), secondary, or monogenic. Ethnic background was categorised according to self-selection, country of birth, parents’ and grandparents’ birthplaces, and the languages spoken at home as European (Anglo-Celtic, southern European, other European) or non-European (Asian, Aboriginal, other).
Ascertainment of monogenic diabetes
For the purposes of this substudy, all patients under 35 years of age at diagnosis were assessed with the UK MODY risk prediction model that is undergoing continuing validation.7 For patients with an initial clinical diagnosis of type 1 diabetes, assuming a pre-test MODY prevalence in such patients of 0.7%, probable MODY is defined as the patient having a greater than 25% probability of MODY, based on family history (a parent with diabetes), sex, age at diagnosis, and glycated haemoglobin (HbA1c) level. For patients with an initial clinical diagnosis of type 2 diabetes, assuming a pre-test MODY prevalence of 4.6%, probable MODY is defined as the patient having a greater than 25% probability of MODY, based on family history (a parent with diabetes), sex, age at diagnosis, BMI, blood glucose-lowering treatment (oral hypoglycaemic agents or insulin), and HbA1c level. We assumed that the initial clinical diagnosis might be incorrect, so both equations were applied to each eligible patient.
Patients identified as being at risk of MODY underwent genetic testing. All coding regions and exon–intron boundaries of the monogenic diabetes genes GCK, HNF1A, HNF4A, HNF1B, NEUROD1, INS, INSR, KCNJ11, ABCC8, PDX1, CEL, PAX6, GATA6, TRMT10A, WFS1, ZFP57, PCBD1, LMNA, PPARG, PLIN1 and POLD1, and the m.3243A > G MIDD mutation were analysed, as well as partial and whole gene deletions and duplications. Targeted next generation sequencing (Agilent custom capture 5.1/Illumina HiSeq; Agilent Technologies LDA UK)10 was performed at the Molecular Genetics Laboratory of the Royal Devon and Exeter NHS Foundation Trust, Exeter, UK.
Ethics approval
The FDS2 was approved by the South Metropolitan Area Health Service Human Research Ethics Committee (reference, 07/397).
Results
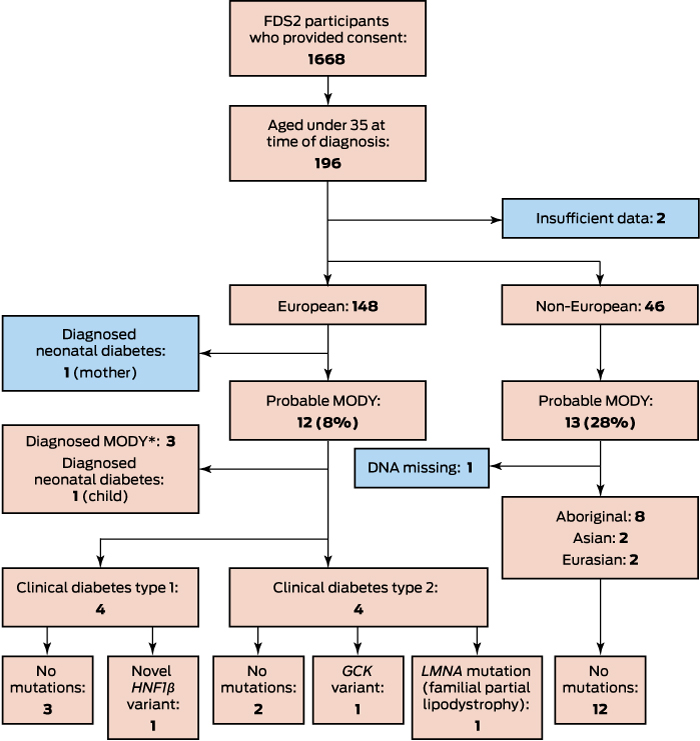
Of 1668 participants, 1499 (89.4%) had been diagnosed with type 2 diabetes, 132 (7.9%) with type 1 diabetes, 12 (0.7%) with LADA, 17 (1.0%) with secondary diabetes, and five (0.3%) with monogenic diabetes (three with MODY, and a mother and child with permanent neonatal diabetes). In total, 196 patients had been diagnosed before age 35 (Box); there were insufficient data for two of these patients to apply the UK MODY risk prediction models, and they were excluded from our analysis. Of the 194 included participants, 148 (76%) were from European and 46 (24%) from non-European ethnic backgrounds.
Of the 148 patients with European ethnic backgrounds who were aged under 35 years at diagnosis, 99 (67%) had clinical diagnoses of type 1 diabetes, 41 (28%) diagnoses of type 2 diabetes, and eight (5%) diagnoses of other types of diabetes, including three with already identified GCK mutations, two with known neonatal diabetes (mother and daughter), one with LADA, and two with secondary diabetes. The three patients with GCK mutations, the younger patient with neonatal diabetes, and eight other patients (a total of 8.1% of participants with European ethnic backgrounds diagnosed before 35 years of age) were classified as at risk of MODY by the UK MODY risk prediction models. Genotyping of the eight patients without confirmed monogenic diabetes identified that one had a GCK mutation, one had a novel, probably non-pathogenic HNF1B variant (c.1207-12T > G), and a third patient had a mutation associated with familial partial lipodystrophy (LMNA, c.1444C > T)11 (Box).
The patient with the HNF1B variant had clinical type 1 diabetes and was receiving insulin pump therapy. As HNF1B variants have been associated with kidney disease,12 renal ultrasonography was performed, but the results were unremarkable. Genotyping of both parents found that the patient’s mother had the same mutation, but neither diabetes nor renal disease. The patient with the LMNA mutation and her father both had clinical features of partial lipodystrophy. The woman with known neonatal diabetes was found to have had diabetes in eastern Europe (at age 33 years) before she moved to Australia; she was diagnosed with permanent neonatal diabetes at the age of 39 years after her daughter had been genotyped.
Of the 46 participants from non-European ethnic backgrounds who were under age 35 years at diagnosis for whom data required for MODY risk prediction were available, 34 (74%) had clinical diagnoses of type 2 diabetes, 10 (22%) of type 1 diabetes, one (2%) of LADA, and one (2%) a diagnosis of secondary diabetes. None had a pre-recruitment diagnosis of monogenic diabetes. The proportion classified as at risk of MODY was higher than for the patients with European ethnic backgrounds (28% v 8%; Box), but none of the 12 patients for whom DNA was available had mutations associated with MODY or permanent neonatal diabetes.
Of 12 patients from European ethnic backgrounds classified by the UK risk prediction models as at risk of MODY, MODY was genotypically confirmed in four (all GCK mutations), a proportion consistent with the greater than 25% risk threshold applied by the models. This yields an overall MODY prevalence among those diagnosed with diabetes in the FDS2 cohort of 0.24% (4 of 1668; 95% confidence interval [CI], 0.08–0.66%). The prevalence of MODY among the 1409 FDS2 patients from European ethnic backgrounds with diagnosed diabetes with valid data was 0.28% (95% CI, 0.09–0.77%); for the 258 from non-European ethnic backgrounds, it was 0.0% (95% CI, 0.0–1.8%).
With regard to prevalence in the general population, we identified 4639 people with diabetes among 157 000 people in the catchment area in 2008.8 If we assume under-ascertainment of 20%,13 this increases the number of people with diabetes to 5799, or a prevalence of 3.7%. This is close to the 3.8% prevalence of self-reported diabetes in Australia reported by the Australian Bureau of Statistics for 2007–2008.14 Assuming that 0.24% of all people with diabetes have MODY (ie, 14 patients among the 157 000 people in the FDS2 catchment area), the overall population prevalence would be 89 cases per million. Similarly, the two cases of neonatal diabetes indicate a prevalence of 0.12% (95% CI, 0.02–0.48%) and an overall population prevalence of 45 cases per million.
Discussion
We found that 0.24% of participants from the community-based FDS2 cohort had MODY that was confirmed by genotyping, with a GKC mutation in each case. One of the four patients with MODY had previously been undiagnosed, but the UK MODY risk prediction models also identified the other three, as well as one of two patients with known permanent neonatal diabetes. All the patients with MODY and neonatal diabetes had European ethnic backgrounds. Although probable MODY was identified in a larger proportion of patients under 35 years of age at diagnosis from non-European ethnic backgrounds, monogenic diabetes was not confirmed by genotyping in any of these people. These data suggest that in multiracial populations, as in Australia, MODY risk prediction as a prelude to genotyping should, as previously acknowledged,7 be applied only to patients with European ethnic backgrounds.
The MODY prevalence of 0.24% was lower than the 0.3–2.4% range reported in the literature.2-4 However, populations in previous studies were predominantly of European ethnic background. The prevalence of MODY mutations was also low in a study of pregnant women in the United States; this finding was primarily attributed to the greater ethnic variety of the sample compared with populations investigated in other epidemiological studies, most of which were from Europe.15 In a UK study of diabetes among Asians aged 0–29 years — among whom, based on age alone, an enrichment of monogenic diabetes cases would be expected — the prevalence of MODY was 0.7%,16 while studies from the Middle East17 and Asia18 have also found that the prevalence of the main mutations associated with MODY may be relatively low among people from non-European ethnic backgrounds. Although these reports might reflect the diluting effect of a higher prevalence of younger onset type 2 diabetes in non-European populations, especially those in south Asia,19 they are nevertheless consistent with our data for young Aboriginal, Asian and Eurasian FDS2 patients identified as being at increased risk of MODY by the UK prediction models, but for whom no relevant mutations were identified by genotyping.
We estimated an overall MODY prevalence in Australia of 89 cases per million population. In European registry-based studies, the prevalence was generally similar (92–108 per million),4,20 but these estimates were regarded as minimum levels, as regional variation in the prevalence of confirmed MODY suggested that rates of genetic testing were heterogeneous. Further, the mildly elevated fasting plasma glucose concentrations associated with GCK mutations may mean that the disorder is suspected only when glycaemic screening is undertaken in a young asymptomatic patient with a strong family history of diabetes, one who is undergoing a medical assessment for work or insurance purposes, or a woman who is pregnant. Although we actively screened for MODY in our FDS2 cohort using risk prediction and targeted genotyping, other people with GCK mutations in the study catchment area may not have been detected, leading to our underestimating the prevalence of MODY in Australians with diabetes.
Differences in MODY phenotypes, and especially the asymptomatic nature of many GCK mutations, may explain why there is substantial variation in the reported relative prevalence of mutations. For the two most frequent MODY types, it has been reported that 16–45% of all patients with MODY carry HNF1A mutations, while the range for GCK mutations is 8–63%.21-23 Our four patients with confirmed MODY all carried GCK mutations, including a mother diagnosed during pregnancy and her teenage son. The only HNF mutation we detected was non-pathogenic. Nevertheless, active identification of MODY is of great clinical importance. Patients with GCK mutations do not usually require pharmacotherapy and do not develop chronic vascular complications,24 and can be overtreated if not diagnosed. Patients with an HNF1A mutation can respond to low doses of sulfonylureas that bypass the molecular defect in the pancreatic β-cell,25 and can also be inappropriately treated with insulin, with the attendant increase in the risk of complications such as hypoglycaemia and weight gain.
Our estimate of the population prevalence of permanent neonatal diabetes (45 cases per million) is higher than the 11 cases per million reported for larger populations.26 The younger of the two patients with this diagnosis in our study was assessed as being at risk of MODY by the UK risk prediction model. Her mother — who was only diagnosed with neonatal diabetes by genotyping and after confirmatory genetic testing of her daughter (prior to FDS2 recruitment), despite having had diabetes for many years — was not identified as being at risk of MODY. Having only two such patients means that our prevalence estimate should be interpreted with caution. Indeed, the relative infrequency of monogenic diabetes and the consequently small numbers of affected patients in the FDS2 cohort is the major limitation of our study. Nevertheless, its strengths include its well characterised, community-based sample and its robust, contemporary genotyping for all testable mutations.
Although our data suggest that only one in 280 Australians diagnosed with diabetes have MODY or permanent neonatal diabetes, the implications of misdiagnosis for the patients and their families can be profound. All clinicians involved in managing patients with diabetes, regardless of clinical type, should therefore be familiar with the features of monogenic diabetes. They should also be aware of the availability of risk prediction tools that can inform the need for genotyping, a relatively expensive test. Consistent with a range of other studies, our data also suggest that young Australians from European ethnic backgrounds may be at greater risk of monogenic diabetes than those from other ethnic groups, including people with an Indigenous Australian background, although the diluting effect of high rates of type 2 diabetes in non-European populations may play a role in this phenomenon. The MODY UK risk prediction models performed well as a prelude to genotyping in Australians from a European ethnic background, but appear to have limited value for assessing people of other ethnicities.
Received 16 October 2016, accepted 6 January 2017
- Timothy ME Davis1
- Ashley E Makepeace2
- Sian Ellard3
- Kevin Colclough4
- Kirsten Peters1
- Andrew Hattersley3
- Wendy A Davis1
- 1 University of Western Australia, Perth, WA
- 2 Fiona Stanley Hospital, Perth, WA
- 3 Institute of Biomedical and Clinical Science, University of Exeter Medical School, Exeter, United Kingdom
- 4 Royal Devon and Exeter NHS Foundation Trust, Exeter, United Kingdom
We are grateful to patients who participated in the Fremantle Diabetes Study Phase II (FDS2), and FDS2 staff for help with collecting and recording clinical information. We thank the biochemistry department at Fremantle Hospital and Health Service for performing laboratory tests. FDS2 has been supported by the National Health and Medical Research Council (NHMRC; project grants 513781 and 1042231). Timothy Davis is supported by an NHMRC Practitioner Fellowship (1058260). The funders had no role in the design and conduct of the study, or in the preparation of the manuscript and the decision to submit it for publication.
No relevant disclosures.
- 1. Murphy R, Ellard S, Hattersley AT. Clinical implications of a molecular genetic classification of monogenic beta-cell diabetes. Nat Clin Pract Endocrinol Metab 2008; 4: 200-213.
- 2. Galler A, Stange T, Muller G, et al. Incidence of childhood diabetes in children aged less than 15 years and its clinical and metabolic characteristics at the time of diagnosis: data from the Childhood Diabetes Registry of Saxony, Germany. Horm Res Paediatr 2010; 74: 285-291.
- 3. Ledermann HM. Maturity-onset diabetes of the young (MODY) at least ten times more common in Europe than previously assumed? Diabetologia 1995; 38: 1482.
- 4. Shields BM, Hicks S, Shepherd MH, et al. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010; 53: 2504-2508.
- 5. Karges B, Meissner T, Icks A, et al. Management of diabetes mellitus in infants. Nat Rev Endocrinol 2012; 8: 201-211.
- 6. Ellard S, Bellanné-Chantelot C, Hattersley AT; European Molecular Genetics Quality Network MODY Group. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia 2008; 51: 546-553.
- 7. Shields BM, McDonald TJ, Ellard S, et al. The development and validation of a clinical prediction model to determine the probability of MODY in patients with young-onset diabetes. Diabetologia 2012; 55: 1265-1272.
- 8. Davis TM, Bruce DG, Davis WA. Cohort profile: the Fremantle Diabetes Study. Int J Epidemiol 2013; 42: 412-421.
- 9. Australian Bureau of Statistics. Socio-economic indexes for areas. Updated Sept 2013. http://www.abs.gov.au/websitedbs/censushome.nsf/home/seifa (accessed Sept 2015).
- 10. Ellard S, Lango Allen H, De Franco E, et al. Improved genetic testing for monogenic diabetes using targeted next-generation sequencing. Diabetologia 2013; 56: 1958-1963.
- 11. Shackleton S, Lloyd DJ, Jackson SN, et al. LMNA, encoding lamin A/C, is mutated in partial lipodystrophy. Nat Genet 2000; 24: 153-156.
- 12. Heidet L, Decramer S, Pawtowski A, et al. Spectrum of HNF1B mutations in a large cohort of patients who harbor renal diseases. Clin J Am Soc Nephrol 2010; 5: 1079-1090.
- 13. Harvey JN, Craney L, Kelly D. Estimation of the prevalence of diagnosed diabetes from primary care and secondary care source data: comparison of record linkage with capture-recapture analysis. J Epidemiol Community Health 2002; 56: 18-23.
- 14. Australian Bureau of Statistics. 4820.0.55.001. Diabetes in Australia: a snapshot, 2007–08. Sept 2011. http://www.abs.gov.au/ausstats/abs@.nsf/mf/4820.0.55.001 (accessed Jan 2017).
- 15. Sewell MF, Presley LH, Holland SH, Catalano PM. Genetic causes of maturity onset diabetes of the young may be less prevalent in American pregnant women recently diagnosed with diabetes mellitus than in previously studied European populations. J Matern Fetal Neonatal Med 2015; 28: 1113-1115.
- 16. Harron KL, Feltbower RG, McKinney PA, et al. Rising rates of all types of diabetes in south Asian and non-south Asian children and young people aged 0–29 years in West Yorkshire, UK, 1991–2006. Diabetes Care 2011; 34: 652-654.
- 17. Al-Sheyab F, Khamaiseh E, Halaweh MA, Khaul RW. Characterization of glucokinase polymorphisms associated with maturity-onset diabetes of the young (MODY2) in Jordanian population. Tsitol Genet 2009; 43: 58-63.
- 18. Ng MC, Cockburn BN, Lindner TH, et al. Molecular genetics of diabetes mellitus in Chinese subjects: identification of mutations in glucokinase and hepatocyte nuclear factor-1α genes in patients with early-onset type 2 diabetes mellitus/MODY. Diabet Med 1999; 16: 956-963.
- 19. Misra S, Shields B, Colclough K, et al. South Asian individuals with diabetes who are referred for MODY testing in the UK have a lower mutation pick-up rate than white European people. Diabetologia 2016; 59: 2262-2265.
- 20. Søvik O, Irgens HU, Molnes J, et al. Monogenic diabetes mellitus in Norway. Norsk Epidemiologi 2013; 23: 55-60.
- 21. Lindner TH, Cockburn BN, Bell GI. Molecular genetics of MODY in Germany. Diabetologia 1999; 42: 121-123.
- 22. Moises RS, Reis AF, Morel V, et al. Prevalence of maturity-onset diabetes of the young mutations in Brazilian families with autosomal-dominant early-onset type 2 diabetes. Diabetes Care 2001; 24: 786-788.
- 23. Chevre JC, Hani EH, Boutin P, et al. Mutation screening in 18 Caucasian families suggest the existence of other MODY genes. Diabetologia 1998; 41: 1017-1023.
- 24. Giuffrida FM, Reis AF. Genetic and clinical characteristics of maturity-onset diabetes of the young. Diabetes Obes Metab 2005; 7: 318-326.
- 25. Pearson ER, Starkey BJ, Powell RJ, et al. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003; 362: 1275-1281.
- 26. Grulich-Henn J, Wagner V, Thon A, et al. Entities and frequency of neonatal diabetes: data from the diabetes documentation and quality management system (DPV). Diabet Med 2010; 27: 709-712.





Abstract
Objective: To determine the prevalence of monogenic diabetes in an Australian community.
Design: Longitudinal observational study of a cohort recruited between 2008 and 2011.
Setting: Urban population of 157 000 people (Fremantle, Western Australia).
Participants: 1668 (of 4639 people with diabetes) who consented to participation (36.0% participation).
Main outcome measures: Prevalence of maturity-onset diabetes of the young (MODY) and permanent neonatal diabetes in patients under 35 years of age, from European and non-European ethnic backgrounds, who were at risk of MODY according to United Kingdom risk prediction models, and who were then genotyped for relevant mutations.
Results: Twelve of 148 young participants with European ethnic backgrounds (8%) were identified by the risk prediction model as likely to have MODY; four had a glucokinase gene mutation. Thirteen of 45 with non-European ethnic backgrounds (28%) were identified as likely to have MODY, but none had a relevant mutation (DNA unavailable for one patient). Two patients with European ethnic backgrounds (one likely to have MODY) had neonatal diabetes. The estimated MODY prevalence among participants with diagnosed diabetes was 0.24% (95% confidence interval [CI], 0.08–0.66%), an overall population prevalence of 89 cases per million; the prevalence of permanent neonatal diabetes was 0.12% (95% CI, 0.02–0.48%) and the population prevalence 45 cases per million.
Conclusions: One in 280 Australians diagnosed with diabetes have a monogenic form; most are of European ethnicity. Diagnosing MODY and neonatal diabetes is important because their management (including family screening) and prognosis can differ significantly from those for types 1 and 2 diabetes.