The known Antimicrobial drug resistance is a global problem, and reducing antibiotic use is the most important clinical response. Australian GPs are reported to overprescribe antibiotics, but the extent has not been quantified.
The new New acute respiratory infections (ARIs) accounted for 51% of all new problems in general practice managed with an antibiotic. Had GPs adhered to widely consulted antibiotic prescribing guidelines, the rate of prescription would have been 11–23% of the current rate (an estimated 0.65–1.36 million prescriptions per year nationally, instead of 5.97 million).
The implications Antibiotic prescribing in Australian general practice could be substantially reduced were GPs to adhere more closely to guideline recommendations.
Acute respiratory infections (ARIs) are among the most frequently managed problems in Australian general practice.1 Antibiotics were once the mainstay of treatment, but it is now recognised that any benefits are often outweighed by their harms (adverse drug reactions, financial costs, antibiotic resistance).2 International guidelines consistently advise against routinely prescribing antibiotics for several ARIs, including acute bronchitis, most cases of acute pharyngitis and acute rhinosinusitis, and influenza.3-5 Diagnostic uncertainty, beliefs of clinicians and patients about antibiotic resistance and antibiotics, and a lack of evidence-based alternative treatments mean, however, that antibiotics continue to be prescribed at high rates.6,7 About 97 million outpatient visits in the United States annually result in antibiotic prescriptions, 41% of them for ARIs.8 Antibiotic consumption is even higher in Australia: in 2010, 87 antibiotic doses (units) were dispensed per person, compared with 22 per person in the US.9 Overprescribing of antibiotics for ARIs in Australian general practice probably contributes to this high rate, and the Australian Strategic and Technical Advisory Group on Antimicrobial Resistance (ASTAG), advising the Australian government, has called for national benchmarks for antibiotic prescribing.10 The level of overprescribing for ARIs in general practice has not been quantified.
We therefore investigated two questions:
-
What is the current rate of antibiotic prescribing by Australian general practitioners for each of eight ARI diagnoses?
-
How much does this rate diverge from the estimated rate of antibiotic prescribing for these ARIs were GPs prescribing them according to the recommendations of the widely accepted Australian prescribing guidelines, Therapeutic Guidelines: Antibiotic and Therapeutic Guidelines: Respiratory?3,5
Methods
We used ARI management data collected in general practice during April 2010 – March 2015 by the Bettering the Evaluation and Care of Health (BEACH) program. BEACH is a continuous cross-sectional survey of the clinical activity of GPs in Australia; its methods have been reported elsewhere.1 In brief, each year a random sample of about 1000 GPs report details for 100 consecutive encounters with patients (with the patients’ consent). The data collected in structured paper forms include the problems managed (classified post hoc by trained clinical coders according to the International Classification of Primary Care, version 2 [ICPC-2]11) and medications prescribed (classified according to the Anatomical Therapeutic Chemical [ATC] classification;12 antibiotics were defined as ATC class J01, “Antibacterials for systemic use”), with direct linkage between the two datasets.1
“Problem” was defined as a statement of the provider’s understanding of the health problem presented by a patient, family or community. GPs are asked to record problems as specifically as possible on the basis of the information available at the time, and the description may be limited to recording symptoms. The problem can be classified as being new or old; we analysed only new problems (“cases” in this article). New problems are defined as the first presentation of a problem, including the first presentation of the recurrence of a previously resolved problem, but excluding the presentation of a problem first assessed by another provider.
ARI diagnoses included
We identified 18 potentially relevant codes in the list of ICPC-2 infections and symptoms/complaints codes collected by BEACH, ten of which we agreed corresponded to ARIs (Box 1); eight symptom codes were excluded because they could be related to non-ARI diagnoses and were used infrequently (online Appendix, table 1). Therapeutic Guidelines: Antibiotic and Therapeutic Guidelines: Respiratory3,5 include nine ARI diagnoses that are defined slightly differently. We assigned one or more of the ten ICPC-2 codes to each guideline diagnosis, resulting in eight included ARI diagnoses (Box 1).
We extracted the number of cases for each diagnosis and the number treated with one or more systemic antibiotics per 100 total encounters for each of the ten ICPC-2 codes in Box 1. This allowed us to estimate the numbers of cases and the number for which antibiotics were prescribed in general practice both per full-time equivalent (FTE) GP (calculated by the Australian Institute of Health and Welfare from hours worked, as collected at annual registration from each GP; assuming an FTE of 40 hours/week) and nationally per year.
Estimated rates of antibiotic prescribing had GPs adhered to guidelines
We extracted the guideline recommendations for the eight ARI diagnoses. If antibiotics are not recommended or the guidelines do not make a recommendation (laryngitis only), we reported the guideline rate as 0%; if antibiotics are always recommended, we reported the guideline rate as 100%; and if recommended should specific criteria be fulfilled (online Appendix, table 2), we estimated the guideline rate as being the proportion of patients with each infection who satisfied the relevant criteria, based on data from the BEACH program1 and primary studies. As there were differences in these proportions between studies, we report minimum and maximum rates.
We identified primary studies for determining the proportions of patients with each infection who satisfied antibiotic use criteria by searching: studies in relevant Cochrane reviews;13-15 PubMed, to identify studies of any design conducted in Australia between 2010 and July 2015 (search strategy: online Appendix, table 3); reference lists of review articles and systematic reviews; and our personal libraries. We contacted authors of primary studies to seek additional data when necessary.
The complete data for calculating guideline estimates are included in the online Appendix, tables 4–6).
Comparing current antibiotic prescribing with guideline recommendations
For each diagnosis, we calculated (nationally, and per FTE GP/year):
-
the current rate and the guideline-recommended rate of new cases that were managed with at least one antibiotic; and
-
the estimated minimum number of excess cases (difference between current mean rate and maximum guideline-recommended rate) for which antibiotics had been prescribed (for an example, see Box 2).
Ethics approval
The BEACH program is approved by the Human Research Ethics Committee of the University of Sydney (reference, 11428; valid until 31 March 2018); prior to March 2011, the BEACH program was also approved by the Ethics Committee of the Australian Institute of Health and Welfare (reference, EC205).
Results
A total of 39 571 new ARI cases were managed at 487 400 encounters during the 5-year study period, a mean of 8.1 new ARI cases per 100 encounters (Box 3). At least one antibiotic was prescribed or supplied by the GP for 22 596 ARI cases (57%; Box 3).
The eight ARIs accounted for 22 596 of 44 434 all new cases (50.9%) managed with at least one antibiotic (for any indication, respiratory or non-respiratory). Acute rhinosinusitis accounted for 20.9% of all problems managed with an antibiotic, acute bronchitis/bronchiolitis for 14.4%, acute otitis media for 6.9%, acute pharyngitis or tonsillitis for 6.2%, community-acquired pneumonia for 1.3%, laryngitis for 0.5%, pertussis for 0.5%, and influenza for 0.2%.
An estimated mean 5.97 million (95% confidence interval [CI], 5.69–6.24 million) ARI cases per year were managed in Australian general practice with at least one antibiotic, equivalent to an estimated 230 cases per FTE GP/year (95% CI, 219–240 cases/FTE/year) or 254 cases per 1000 population.
Antibiotics are not recommended by Therapeutic Guidelines for acute bronchitis/bronchiolitis (current prescribing rate, 85%), nor for influenza (11%) unless complicated by secondary bacterial pneumonia, in which circumstance the recommendation for community-acquired pneumonia applies. Antibiotics are always recommended for community-acquired pneumonia (current prescribing rate, 72%) and pertussis (71%); and they are recommended for 0.5–8% of cases of acute rhinosinusitis (current prescribing rate, 41%), 20–31% of cases of acute otitis media (89%), and 19–40% cases of acute pharyngitis or tonsillitis (94%) (Box 3). Therapeutic Guidelines do not make a recommendation about treating laryngitis with antibiotics (current rate of prescribing, 21%).
Had they adhered to guideline recommendations, GPs would have managed 6–13% of new cases of ARI with an antibiotic, equivalent to 0.65–1.36 million cases each year, or 25–52 cases per FTE GP (Box 3); this rate is 11–23% of the current actual rate. Antibiotics were prescribed more frequently than recommended by guidelines for acute rhinosinusitis, acute bronchitis/bronchiolitis, acute otitis media, and acute pharyngitis or tonsillitis (Box 4).
Discussion
In this sample of Australian GPs, an estimated 5.97 million new cases of ARI per year accounted for half of all new problems managed with an antibiotic, equivalent to an estimated 230 cases per FTE GP/year. Three studies that investigated the prescribing of antibiotics for ARIs in Australian general practice reported similar findings; their results differed slightly because different diagnostic codes were included.19-21 A study in the US found a similar overall antibiotic prescribing rate for all conditions (221 per 1000 population)22 to our Australian data (254 per 1000). The US report found antibiotics were overprescribed for ARIs by about 100% when compared with guideline recommendations;22 our estimates suggest that overprescribing is greater in Australia (by 148–418%). Possible explanations for the different estimates by these studies include differences in diagnostic labels and guideline recommendations. Unlike the American guidelines,4 for example, Australian guidelines do not recommend testing for group A streptococcal infections in patients presenting with acute pharyngitis or tonsillitis, but both guidelines nevertheless suggest that up to 40% of these patients should be treated with antibiotics.22
Our findings suggest that antibiotics are overprescribed for treating patients with ARIs. Diagnostic uncertainty — concern by the treating doctor that a serious infection or complication might be missed — is one potential explanation for this finding;6 for example, clinicians overestimate the rate of infectious complications of acute otitis media (mastoiditis) and tonsillitis (peritonsillar abscess).23 Data from a high quality cohort study in the United Kingdom found that reducing the rate of antibiotic prescribing was not associated with increased rates of mastoiditis, empyema, bacterial meningitis, or intracranial abscess.24 The investigators found a slight increase in the rates of pneumonia and quinsy, but the effect size was small; each 10% reduction in antibiotic prescribing was associated with one additional case of pneumonia per year and one case of quinsy per decade per practice of 7000 patients.24
In our study, antibiotics were prescribed slightly less frequently than recommended for community-acquired pneumonia and pertussis. Possible explanations for these unexpected findings could be that GPs refer patients with community-acquired pneumonia to hospital, but manage patients with cough that persists for more than 3 weeks but with only a weak presumption of pertussis.3
Our estimates of guideline-recommended rates of antibiotic prescribing are not tailored to specific types of patient or practice. To increase the generalisability of our findings, we extracted antibiotic prescribing recommendations from widely used Australian prescribing guidelines and, as far as possible, used Australian data on the proportions of participants who met the prescribing criteria in these guidelines. Therapeutic Guidelines are probably the most consulted guidelines for antibiotic prescribing in Australian general practice, but access requires a paid subscription, possibly a barrier to their broadest possible implementation.
We obtained data on antibiotic prescribing from the BEACH cross-sectional study of Australian general practice activity. BEACH diagnosis and treatment data are considered robust: clinicians record the problems managed or diagnosed at each encounter in free text, and a team of trained clinical coders assigns appropriate ICPC-2 codes. However, clinicians may record diagnoses they believe warrant treatment with antibiotics more than those that do not,25 by recording for example, “community-acquired pneumonia” rather than “acute bronchitis/bronchiolitis”. This, however, seems unlikely, given the low rates of community-acquired pneumonia recorded. Participating clinicians are also asked to record the problem at the highest level possible on the basis of the available evidence; ie, an acute exacerbation of chronic obstructive pulmonary disease (COPD) should be recorded as “COPD” rather than “acute bronchitis”. It is possible that such cases were misclassified, but the effect would probably be small.
Rates of antibiotic prescribing may not reflect the volume of antibiotics dispensed or consumed. Delayed prescriptions (“wait-and-see” prescriptions) might be provided by GPs; Therapeutic Guidelines: Antibiotic specifically recommend delayed prescriptions for children over 6 months old with acute otitis media and no systemic features.3 The proportion of such prescriptions has not been assessed in Australia. Further, patients may themselves choose not to take the antibiotics prescribed by their doctor.
Data collected by the BEACH study may have been subject to selection bias. If GPs who adhered to guidelines were more likely to participate in BEACH because they were less likely to avoid scrutiny, actual antibiotic prescribing rates may have been underestimated.
Conclusion
Antibiotics are prescribed for ARIs at rates 4–9 times as high as those recommended by clinical guidelines. The potential for reducing rates of antibiotic prescription and to thereby reduce rates of antibiotic-related harms, particularly bacterial resistance, is therefore substantial. Our data provide the basis for setting absolute targets for reducing antibiotic prescribing in Australian general practice.
Box 1 – Acute respiratory infection diagnoses and International Classification of Primary Care, version 2 (ICPC-2) codes
|
Acute respiratory infection diagnoses* |
ICPC-2 codes and rubric labels |
||||||||||||||
|
|
|||||||||||||||
|
Acute rhinosinusitis |
R74: Upper respiratory infection acute |
||||||||||||||
|
Acute bronchitis/acute bronchiolitis† |
R78: Acute bronchitis/bronchiolitis |
||||||||||||||
|
Acute otitis media |
H71: Acute otitis media/myringitis |
||||||||||||||
|
Acute pharyngitis and/or tonsillitis |
R72: Strep throat |
||||||||||||||
|
Community-acquired pneumonia |
R81: Pneumonia |
||||||||||||||
|
Pertussis |
R71: Whooping cough |
||||||||||||||
|
Laryngitis‡ |
R77: Laryngitis/tracheitis acute |
||||||||||||||
|
Influenza |
R80: Influenza |
||||||||||||||
|
|
|||||||||||||||
|
* From Therapeutic Guidelines: Antibiotic3 unless otherwise stated. † Acute bronchitis is listed in Therapeutic Guidelines: Respiratory,5 and was conflated in BEACH with acute bronchiolitis; the two share an ICPC-2 code, and we used the ICPC-2 descriptor. ‡ Laryngitis is not listed in Therapeutic Guidelines: Antibiotic.3 |
|||||||||||||||
Box 2 – Calculating current general practice rates and recommended guideline rates of antibiotic prescribing, and of the minimum rate of antibiotic overprescribing. Example: new cases of acute rhinosinusitis
|
|
|||||||||||||||
|
[a] Mean number of Medicare GP items of service claimed per year in Australia, 2010–2015 (“encounters”)16 |
128.7 million |
||||||||||||||
|
[b] Full-time equivalent (FTE) Australian GPs*17 |
25 991 |
||||||||||||||
|
[c] Mean number of new cases (95% CI) per 100 encounters† |
4.65 (4.53–4.78) |
||||||||||||||
|
[d] Mean estimated number of new cases (95% CI) nationally/year (= [a] × [c]/100) |
5.99 million (5.83–6.15 million) |
||||||||||||||
|
[e] Mean estimated number of new cases (95% CI) per FTE GP/year (= [d]/[b]) |
230 (224–237) |
||||||||||||||
|
Current antibiotic prescribing rates |
|||||||||||||||
|
[f] Mean percentage of new cases (95% CI) managed with an antibiotic† |
40.9% (39.7–42.0%) |
||||||||||||||
|
[g] Mean estimated number of new cases (95% CI) managed with an antibiotic nationally/year (= [d] × [f]) |
2.45 million (2.35–2.54 million) |
||||||||||||||
|
[h] Mean estimated number of new cases managed with antibiotic (95% CI) per FTE GP/year (= [e] × [f]) |
94.1 (90.5–97.8) |
||||||||||||||
|
Antibiotic prescribing, according to guidelines |
|||||||||||||||
|
[i] Percentage of new cases that would be managed with antibiotic according to guidelines (range)‡ |
0.5–8% |
||||||||||||||
|
[j] Estimated number of new cases that would be managed with antibiotic according to guidelines nationally/year (range) (= [d] × [i]) |
0.03–0.48 million |
||||||||||||||
|
[k] Estimated number of new cases that would be managed with antibiotic according to guidelines per FTE GP/year (range) (= [e] × [i]) |
1–18 |
||||||||||||||
|
Estimated minimum excess number of new acute rhinosinusitis cases for which antibiotics were prescribed§ |
|||||||||||||||
|
[l] In general practice nationally/year (= [g] – max[j]) |
2.0 million |
||||||||||||||
|
[m] per FTE GP/year (= [h] – max[k]) |
76 |
||||||||||||||
|
|
|||||||||||||||
|
* 26 885 GPs working a mean 38.6 hours/week = 110.6 FTEs per 100 000 population. FTE is based on a standard working week of 40 hours; total FTE GPs = FTE rate (110.6 per 100 000) × 23.5 million (Australian population, 2014).18 † BEACH data.1 ‡ Based on prescribing criteria in online Appendix, table 4. § Difference between the mean BEACH data estimate of current antibiotic prescribing rate and the maximum percentage guideline-recommended rate. |
|||||||||||||||
Box 3 – Estimated general practice rates (BEACH data, April 2010 – March 2015) and recommended guideline rates of antibiotic prescribing for selected new cases of acute respiratory infection (ARI)*
|
Diagnosis, and guideline criteria for antibiotic prescribing |
Mean number of new cases |
Mean number of new cases managed with antibiotics: current practice |
Mean number of new cases to be managed with antibiotics: guideline recommendations |
||||||||||||
|
per 100 encounters |
per FTE GP/year |
per year, millions |
% cases† |
per 100 encounters |
per FTE GP/year |
per year |
% cases (range) |
per FTE GP/year |
|||||||
|
|
|||||||||||||||
|
Acute rhinosinusitis |
4.65 |
230 |
2.45 |
41% |
1.90 |
94 |
0.03–0.48 |
0.5–8% |
1–18 |
||||||
|
Has bacterial sinusitis |
|
|
|
|
|
|
|
0.5–8% |
|
||||||
|
Acute bronchitis/bronchiolitis |
1.54 |
76 |
1.70 |
85% |
1.32 |
65 |
0 |
0 |
0 |
||||||
|
Acute otitis media |
0.71 |
35 |
0.81 |
89% |
0.63 |
31 |
0.19–0.29 |
20–31% |
7–11 |
||||||
|
Indigenous Australian‡ |
|
|
|
|
|
|
|
3% |
|
||||||
|
< 6 months old‡ |
|
|
|
|
|
|
|
1% |
|
||||||
|
Systemic features (high fever, vomiting or lethargy)§ |
|
|
|
|
|
|
|
12–13% |
|
||||||
|
Persisting symptoms or worse at day 2§ |
|
|
|
|
|
|
|
5–14% |
|
||||||
|
Acute pharyngitis/tonsillitis |
0.60 |
30 |
0.73 |
94% |
0.56 |
28 |
0.14–0.31 |
19–40% |
6–12 |
||||||
|
Indigenous, 2–25 years old‡ |
|
|
|
|
|
|
|
2% |
|
||||||
|
Has rheumatic heart disease¶ |
|
|
|
|
|
|
|
0.03–0.18% |
|
||||||
|
Has scarlet fever¶ |
|
|
|
|
|
|
|
0.18% |
|
||||||
|
Has at least three of high fever; tender large cervical lymph nodes; tonsillar exudate; no cough¶ |
|
|
|
|
|
|
|
17–39% |
|
||||||
|
Community-acquired pneumonia |
0.16 |
8 |
0.15 |
72% |
0.12 |
6 |
0.2 |
100% |
8 |
||||||
|
Pertussis |
0.06 |
3 |
0.05 |
71% |
0.04 |
2 |
0.08 |
100% |
3 |
||||||
|
Laryngitis |
0.20 |
10 |
0.06 |
21% |
0.04 |
2 |
NR |
NR |
NR |
||||||
|
Influenza |
0.19 |
9 |
0.03 |
11% |
0.02 |
1 |
0 |
0 |
0 |
||||||
|
All acute respiratory infections |
8.11 |
402 |
5.97 |
57% |
4.64 |
230 |
0.65–1.36 |
6–13% |
25–52 |
||||||
|
|
|||||||||||||||
|
NR = no recommendation in Therapeutic Guidelines: Antibiotic.3 * Data have been rounded for purposes of presentation. † Extracted from BEACH data.1 ‡ Therapeutic Guidelines: Antibiotic recommends antibiotics for patients with the relevant ARI who fulfil these criteria. The proportions of new cases of each ARI that fulfil these criteria presenting to Australian general practice were extracted from BEACH data. § The proportions of new cases of each ARI that fulfil these criteria were extracted from references in online Appendix, table 6. ¶ The proportions of new cases of each ARI that fulfil these criteria were extracted from references in online Appendix, table 5. |
|||||||||||||||
Box 4 – Numbers of new acute respiratory infection (ARI) cases managed with antibiotics each year in Australia: current practice and estimated maximum guideline-recommended rates. A. Numbers of new cases, nationally; B. New cases per full-time equivalent (FTE) general practitioner
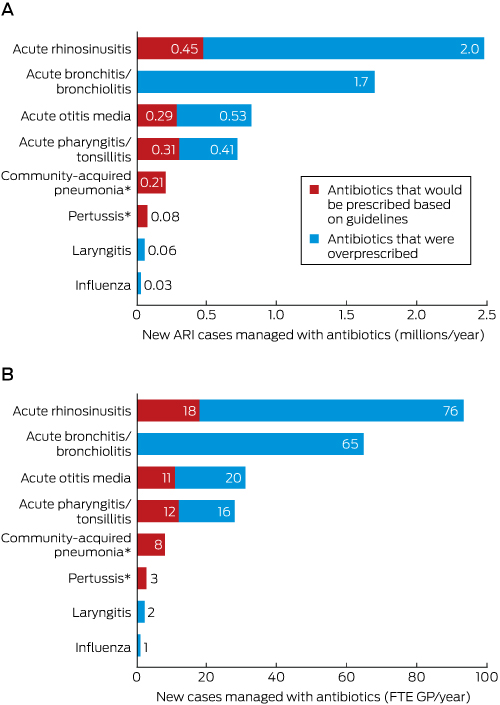
Numbers have been rounded for clarity of presentation. * The numbers of cases of community-acquired pneumonia and pertussis managed with antibiotics were lower than the estimated number recommended by the guidelines.
Received 31 August 2016, accepted 14 March 2017
- Amanda R McCullough1
- Allan J Pollack2
- Malene Plejdrup Hansen3
- Paul P Glasziou1
- David FM Looke4
- Helena C Britt5
- Christopher B Del Mar6
- 1 Centre for Research in Evidence-Based Practice, Bond University, Gold Coast, QLD
- 2 Family Medicine Research Centre, University of Sydney, Sydney, NSW
- 3 Research Unit for General Practice, Aalborg University, Aalborg, Denmark
- 4 Princess Alexandra Hospital, Brisbane, QLD
- 5 University of Sydney, Sydney, NSW
- 6 Bond University, Gold Coast, QLD
This investigation was supported by the Centre for Research Excellence in Minimising Antibiotic Resistance from Acute Respiratory Infections, funded by the National Health and Medical Research Council (1044904).
Between April 2010 and March 2015, the BEACH program was funded by the Australian Government Department of Health and Ageing, the Australian Government Department of Veterans’ Affairs, AstraZeneca (Australia), bioCSL (Australia), Novartis Pharmaceuticals Australia, AbbVie, Merck, Sharp and Dohme (Australia), Pfizer Australia, GlaxoSmithKline Australia, Sanofi-Aventis Australia, Bayer Australia, and the National Prescribing Service. The funding bodies did not influence the concept, design or conduct of the research, nor the preparation of this article; no financial support was provided for preparing the manuscript. Christopher Del Mar has received funding (personal and institutional) from the Australian Commission for Safety and Quality in Health Care (ACSQHC) and British United Provident Association (BUPA) for consulting (regarding shared decision making).
- 1. Britt H, Miller G, Henderson J, et al. General practice activity in Australia: 2013–14 (General Practice Series No. 37). Sydney: Sydney University Press, 2014. https://ses.library.usyd.edu.au/bitstream/2123/11882/4/9781743324226_ONLINE.pdf (accessed Apr 2017).
- 2. Smith S, Fahey T, Smucny J, Becker L. Antibiotics for acute bronchitis. Cochrane Database Syst Rev 2014; (3): CD000245.
- 3. Antibiotic Expert Groups. Therapeutic guidelines: antibiotic, version 15. Melbourne: Therapeutic Guidelines, 2014. https://tgldcdp.tg.org.au/guideLine?guidelinePage=Antibiotic&frompage=etgcomplete (accessed May 2017).
- 4. Harris AM, Hicks LA, Qaseem A, et al. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2016; 164: 425-434.
- 5. Respiratory Expert Groups. Therapeutic guidelines: respiratory, version 5. Melbourne: Therapeutic Guidelines, 2014. https://tgldcdp.tg.org.au/guideLine?guidelinePage=Respiratory&frompage=etgcomplete (accessed May 2017).
- 6. Tonkin-Crine S, Yardley L, Little P. Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. J Antimicrob Chemother 2011; 66: 2215-2223.
- 7. McCullough A, Rathbone J, Parekh S, et al. Not in my backyard: a systematic review of clinicians’ knowledge and beliefs about antibiotic resistance. J Antimicrob Chemother 2015; 70: 2465-2473.
- 8. Shapiro DJ, Hicks LA, Pavia AT, Hersh AL. Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. J Antimicrob Chemother 2014; 69: 234-240.
- 9. Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis 2014; 14: 742-750.
- 10. Australian Commission on Safety and Quality in Health Care. Australian atlas of healthcare variation [website]. 2015. http://www.safetyandquality.gov.au/atlas/ (accessed Dec 2016).
- 11. Classification Committee of the World Organization of Family Doctors (WONCA). ICPC-2. International Classification of Primary Care, 2nd ed. Oxford: Oxford University Press, 1998.
- 12. WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2014. 17th edition. Oslo: WHO-CCDSM, Norwegian Institute of Public Health, 2013. http://www.sifac.it/sites/default/files/dice1838.pdf (accessed Dec 2016).
- 13. Venekamp R, Sanders S, Glasziou P, et al. Antibiotics for acute otitis media in children. Cochrane Database Syst Rev 2013; (1): CD000219.
- 14. Spinks A, Glasziou PP, Del Mar CB. Antibiotics for sore throat. Cochrane Database Syst Rev 2013; (11): CD000023.
- 15. Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database Syst Rev 2013; (6): CD000247.
- 16. Britt H, Miller GC, Henderson J, et al. A decade of Australian general practice activity: 2005–06 to 2014–15 (General Practice Series No. 39). Sydney: Sydney University Press, 2015. https://ses.library.usyd.edu.au/bitstream/2123/13974/4/9781743324554_ONLINE.pdf (accessed Apr 2017).
- 17. Australian Institute of Health and Welfare. Medical workforce 2014: additional material. http://www.aihw.gov.au/workforce/medical/2014/additional/ (accessed Apr 2017).
- 18. Australian Bureau of Statistics. Australian demographic statistics, Jun 2015 [website]. Dec 2015. http://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/6CBA90A25BAC951DCA257F7F001CC559?opendocument (accessed Apr 2017).
- 19. Pan Y, Henderson J, Britt H. Antibiotic prescribing in Australian general practice: how has it changed from 1990–91 to 2002–03? Respir Med 2006; 100: 2004-2011.
- 20. Britt H, Harrison C, Miller G. The real story, GP prescribing of antibiotics for respiratory tract infections — from BEACH. Byte from BEACH [online] 2012; (2). http://sydney.edu.au/medicine/fmrc/beach/bytes/BEACH-Byte-2012-002.pdf (accessed Apr 2017).
- 21. Magin PJ, Morgan S, Tapley A, et al. Changes in early-career family physicians‘ antibiotic prescribing for upper respiratory tract infection and acute bronchitis: a multicentre longitudinal study. Fam Pract 2016; 33: 360-367.
- 22. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010–2011. JAMA 2016; 315: 1864-1873.
- 23. Grossman Z, del Torso S, Hadjipanayis A, et al. Antibiotic prescribing for upper respiratory infections: European primary paediatricians’ knowledge, attitudes and practice. Acta Paediatr 2012; 101: 935-940.
- 24. Gulliford MC, Moore MV, Little P, et al. Safety of reduced antibiotic prescribing for self limiting respiratory tract infections in primary care: cohort study using electronic health records. BMJ 2016; 354: i3410.
- 25. Howie JG. Diagnosis — the Achilles heel? J R Coll Gen Pract 1972; 22: 310-315.





Abstract
Objective: To compare the current rate of antibiotic prescribing for acute respiratory infections (ARIs) in Australian general practice with the recommendations in the most widely consulted therapeutic guidelines in Australia (Therapeutic Guidelines).
Design and setting: Comparison of general practice activity data for April 2010 – March 2015 (derived from Bettering the Evaluation and Care of Health [BEACH] study) with estimated rates of prescribing recommended by Therapeutic Guidelines.
Main outcome measures: Antibiotic prescribing rates and estimated guideline-recommended rates per 100 encounters and per full-time equivalent (FTE) GP per year for eight ARIs; number of prescriptions nationally per year.
Results: An estimated mean 5.97 million (95% CI, 5.69–6.24 million) ARI cases per year were managed in Australian general practice with at least one antibiotic, equivalent to an estimated 230 cases per FTE GP/year (95% CI, 219–240 cases/FTE/year). Antibiotics are not recommended by the guidelines for acute bronchitis/bronchiolitis (current prescribing rate, 85%) or influenza (11%); they are always recommended for community-acquired pneumonia (current prescribing rate, 72%) and pertussis (71%); and they are recommended for 0.5–8% of cases of acute rhinosinusitis (current prescribing rate, 41%), 20–31% of cases of acute otitis media (89%), and 19–40% cases of acute pharyngitis or tonsillitis (94%). Had GPs adhered to the guidelines, they would have prescribed antibiotics for 0.65–1.36 million ARIs per year nationally, or at 11–23% of the current prescribing rate. Antibiotics were prescribed more frequently than recommended for acute rhinosinusitis, acute bronchitis/bronchiolitis, acute otitis media, and acute pharyngitis/tonsillitis.
Conclusions: Antibiotics are prescribed for ARIs at rates 4–9 times as high as those recommended by Therapeutic Guidelines. Our data provide the basis for setting absolute targets for reducing antibiotic prescribing in Australian general practice.