Cervical cancer is the most common cancer affecting women in developing countries. It is caused by persistent infection with specific types of human papillomavirus (HPV).1 Quadrivalent human papillomavirus (4vHPV) vaccine is a recombinant vaccine administered as a three-dose course to provide protection against four types of HPV (6, 11, 16 and 18).2 The vaccine is highly efficacious for the four included types, of which 16 and 18 are reported to cause 70% of cervical cancers and 6 and 11 cause anogenital warts.1,3 4vHPV vaccination was introduced under the Australian National Immunisation Program (NIP) in April 2007 for adolescent girls, with an initial catch-up program including women up to 26 years of age. The current ongoing funded program is only for girls in the first year of high school (aged 12–13 years). Recent data suggest that the 4vHPV vaccination program has caused a rapid decline in genital wart presentations in females,4,5 and there are early indications of a reduction in high-grade cervical dysplasias.6
Following advice from the Australian Technical Advisory Group on Immunisation, vaccination of males was recommended as a cost-effective intervention by the Pharmaceutical Benefits Advisory Committee in November 2011.7 Accordingly, 4vHPV vaccination for boys has been added to the Australian NIP, commencing in 2013 and targeting boys aged 12–13 years in a school-based program, with a catch-up program over 2 years for boys aged 14–16 years.7,8 The program aims to reduce the incidence of HPV disease in males, such as anogenital warts and anal intraepithelial neoplasia,9 and reduce sexual pathways of virus transmission. Australia will be the first nation to implement HPV vaccination for boys in a national program.
Vaccines, as with any medicine, have potential adverse reactions varying from mild and expected to rare and/or serious events. Vaccination may cause such events — the nature of adverse events following immunisation (AEFI) and the timing of onset after vaccination are important factors when assessing causation. Adverse events may also coincide temporally with vaccine administration by chance. To interpret postlicensure surveillance data, it is useful to know the background rates of common and rare potential adverse events before introduction of the vaccine.10,11 With this understanding, increases above background rates can be rapidly identified, which can assist with the evaluation and reporting of potential vaccine-associated adverse event rates.
The mass school-based introduction of female 4vHPV vaccination raised a number of well publicised initial safety concerns, including “scares” regarding potential episodes of anaphylaxis and multiple sclerosis after vaccination.12-14 In addition, a mass psychogenic reaction was seen in a Melbourne school vaccination environment,15 with syncope and syncopal seizures occurring in response to the vaccination process.16 Such spurious events may arise from the psychological impact of the vaccination process, particularly when using mass vaccination strategies in a school-based teenaged population.
Two statewide Victorian datasets were accessed — the Victorian Admitted Episodes Dataset (VAED; hospital discharge data) and the Victorian Emergency Minimum Dataset (VEMD; emergency department visit data) — both of which include International Classification of Diseases 10th revision Australian modification (ICD-10-AM) codes. The data included a unique identifier that enabled linking of individuals across the datasets, but were otherwise non-identifying, according to Victorian Department of Health data linkage protocols.17 Ethics approval for the study was provided by the VAED and VEMD data custodians.
The data that we analysed comprised all episodes that occurred in boys aged 12 to < 16 years and were recorded in the VAED and/or VEMD with one of the ICD-10-AM codes listed in Box 1 and an admission or presentation date from 1 July 2004 to 30 June 2009.18 Conditions selected for inclusion are rare adverse events, conditions that patients are likely to present to hospitals with after vaccination, and conditions that have previously been raised as potential sources of concern in Australia and overseas.10,19
We calculated background annual incidence rates as the number of events during the 5-year study period divided by the population at risk during this period, using Australian Bureau of Statistics 2006 mid-year resident population data for males.20
The numbers of and incidence rates for potential AEFI in boys aged 12 to < 16 years are shown in Box 2, and the estimated numbers of cases of potential AEFI per 100 000 adolescent boys that would occur, even in the absence of vaccine, are shown in Box 3. Assuming an 80% vaccination rate with three doses per person — which equates to about 480 000 boys vaccinated and a total of 1 440 000 doses administered nationally per year in the first 2 years of the program — about 2.4 episodes of Guillain-Barré syndrome would be expected to occur within 6 weeks of vaccination. In addition, about 3.9 seizures and 6.5 acute allergy presentations would be expected to occur within 1 day of vaccination, including 0.3 episodes of anaphylaxis.
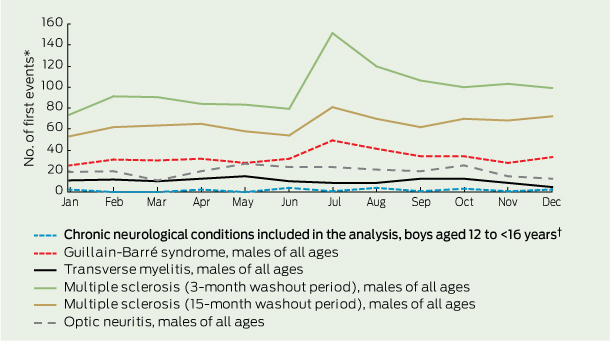
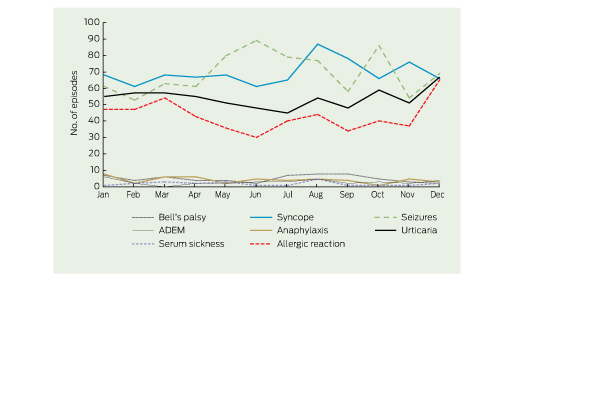
There was minimal seasonal variation in the occurrence of potential AEFI (Box 4, Box 5). However, repeating this analysis with a larger number of neurological presentations (using data for all age groups) revealed a notable peak in the number of multiple sclerosis presentations in July. This peak was reduced but not eliminated when the washout period was increased to 15 months (Box 4).
Using statewide morbidity data, we estimated background rates of neurological and allergic events in adolescent boys in Victoria to be 252.9 and 175.2 per 100 000 person-years, respectively. Such adverse events may be mistakenly assumed to be caused by vaccination, owing to temporal association, when the 4vHPV vaccination program is expanded to include adolescent boys.10 Postlicensure safety assessments of 4vHPV vaccine programs in adolescent girls have shown little evidence of increased risk of neurological and allergic adverse events after vaccination.3,21,22
Expected rates of potential AEFI in recent studies vary widely, but direct comparisons are restricted because of differences in methods, health care systems and data collection and analyses.10,11,23 In particular, caution is required when using emergency presentation databases as these may record preliminary diagnoses, rather than final diagnoses. Studies limited to analysis of ICD-10 coded data, such as ours, lack the rigour of diagnosis verification and conformity to standardised case definitions, although coding standards are maintained. Our study identified higher reporting rates for anaphylaxis compared with similar studies.10,11 While data aberrations are possible, marked increases in anaphylaxis rates Australia and the United States over the past two decades may play a part.24,25
Background rates of potential AEFI and consequent thresholds for safety flags should not be informed merely using data on adolescent girls because sex-related differences could cause misinterpretation of potential signals.10,11 For example, the rate of adolescent boys presenting with a first multiple sclerosis event in the 6 weeks following vaccination would be expected to be one-third of the rate seen for adolescent girls assuming no relationship with vaccine other than temporal.26
In Victoria, first-dose 4vHPV vaccine coverage for adolescent girls has reached 80%,27 but challenges of uptake and course completion by males may be anticipated.28 If coverage for boys is less than 80%, the expected rates in our study should be recalculated to avoid erroneous alert thresholds.
The background rates of potential AEFI that we have estimated can be used to inform surveillance systems, health care providers and the community regarding health care events that may be temporally related to vaccination. In mass vaccination programs, where vaccine exposure is a common event in the target group, many incident acute health conditions will occur following vaccination, irrespective of causal association. While current passive surveillance system reporting is likely to underascertain postvaccination events, prior knowledge of expected numbers of events are valuable in helping determine whether reports or clusters of reports represent real safety flags that require urgent investigation.26
Our data highlight the value of statewide and nationwide health datasets in providing information that can improve public safety. In addition to establishing background rates of diseases, international systems such as those in Denmark and the US, have been used to link vaccination databases to health care event databases, enabling direct investigation of potential associations with adverse events.29-31 These methods, conducted in accordance with state and federal privacy protections, offer a promising future for further improving vaccine safety in Australia.32
2 Numbers of and incidence rates for potential AEFI in boys aged 12 to < 16 years (Victoria, July 2004 – June 2009)
AEFI = adverse events following immunisation. ADEM = acute disseminated encephalomyelitis. |
|||||||||||||||
3 Estimated numbers of cases of potential AEFI in vaccinated boys aged 12 to < 16 years, assuming no relationship with vaccine*
No. of first events per 100 000 population |
No. of episodes per 100 000 population |
||||||||||||||
Received 29 November 2012, accepted 10 March 2013
- Hazel J Clothier1,2
- Katherine J Lee3
- Vijaya Sundararajan4
- Jim P Buttery1,5
- Nigel W Crawford1,6
- 1 Surveillance of Adverse Events Following Vaccination in the Community (SAEFVIC), Murdoch Childrens Research Institute, Melbourne, VIC.
- 2 School of Population and Global Health, University of Melbourne, Melbourne, VIC.
- 3 Clinical Epidemiology and Biostatistics Unit, Murdoch Childrens Research Institute, Melbourne, VIC.
- 4 Department of Medicine, Monash University, Melbourne, VIC.
- 5 Department of Infectious Diseases and Department of Paediatrics, Monash University, Melbourne, VIC.
- 6 Department of General Medicine, Royal Children’s Hospital, Melbourne, VIC.
We thank Lalitha Sundaresan of the Victorian Department of Health for initial data extractions.
Hazel Clothier and Jim Buttery are members of the Advisory Committee on the Safety of Vaccines.
- 1. Cutts FT, Franceschi S, Goldie S, et al. Human papillomavirus and HPV vaccines: a review. Bull World Health Organ 2007; 85: 719-726.
- 2. McCormack PL, Joura EA. Quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine (Gardasil®): a review of its use in the prevention of premalignant genital lesions, genital cancer and genital warts in women. Drugs 2010; 70: 2449-2474.
- 3. Lu B, Kumar A, Castellsagué X, Giuliano AR. Efficacy and safety of prophylactic vaccines against cervical HPV infection and diseases among women: a systematic review and meta-analysis. BMC Infect Dis 2011; 11: 13.
- 4. Fairley CK, Hocking JS, Gurrin LC, et al. Rapid decline in presentations of genital warts after the implementation of a national quadrivalent human papillomavirus vaccination programme for young women. Sex Transm Infect 2009; 85: 499-502.
- 5. Donovan B, Franklin N, Guy R, et al. Quadrivalent human papillomavirus vaccination and trends in genital warts in Australia: analysis of national sentinel surveillance data. Lancet Infect Dis 2011; 11: 39-44.
- 6. Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53 Suppl 1: S29-S35.
- 7. Pharmaceutical Benefits Advisory Committee. November 2011 PBAC meeting outcomes — positive recommendations. http://www. health.gov.au/internet/main/publishing.nsf/Content/DF3D2BF61025D73CCA25796600 80CA50/$File/PBAC%20Outcomes%20Nov% 202011%20-%20Positive%20recommen dations.pdf (accessed Feb 2013).
- 8. Department of Health and Ageing. HPV vaccine extended to boys [media release]. 12 Jul 2012. http://www.health.gov.au/internet/ministers/publishing.nsf/Content/mr-yr12-tp-tp059.htm (accessed Feb 2013).
- 9. Hillman RJ, Giuliano AR, Palefsky JM, et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol 2012; 19: 261-267.
- 10. Siegrist CA, Lewis EM, Eskola J, et al. Human papilloma virus immunization in adolescent and young adults: a cohort study to illustrate what events might be mistaken for adverse reactions. Pediatr Infect Dis J 2007; 26: 979-984.
- 11. Callréus T, Svanström H, Nielsen NM, et al. Human papillomavirus immunisation of adolescent girls and anticipated reporting of immune-mediated adverse events. Vaccine 2009; 27: 2954-2958.
- 12. Gold MS, McIntyre P. Human papillomavirus vaccine safety in Australia: experience to date and issues for surveillance. Sex Health 2010; 7: 320-324.
- 13. Chang J, Campagnolo D, Vollmer TL, Bomprezzi R. Demyelinating disease and polyvalent human papilloma virus vaccination. J Neurol Neurosurg Psychiatry 2011; 82: 1296-1298.
- 14. Brotherton JM, Gold MS, Kemp AS, et al. Anaphylaxis following quadrivalent human papillomavirus vaccination. CMAJ 2008; 179: 525-533.
- 15. Buttery JP, Madin S, Crawford NW, et al. Mass psychogenic response to human papillomavirus vaccination. Med J Aust 2008; 189: 261-262. <MJA full text>
- 16. Crawford NW, Clothier HJ, Elia S, et al. Syncope and seizures following human papillomavirus vaccination: a retrospective case series. Med J Aust 2011; 194: 16-18. <MJA full text>
- 17. Department of Health (Victoria). Victorian data linkages. http://www.health.vic.gov.au/vdl (accessed Feb 2013).
- 18. National Centre for Classification in Health. International statistical classification of diseases and related health problems. 10th revision, Australian modification (ICD-10-AM). Sydney: NCCH, 2008. http://meteor.aihw.gov.au/content/index.phtml/itemId/360927 (accessed Feb 2013).
- 19. Clothier H, Lee K, Crawford N, et al. Background rates of conditions that may present as potential adverse events following H1N1 vaccination in Australia [abstract]. 28th Annual Meeting of the European Society of Paediatric Infectious Diseases; 2010 May 4-8; Nice, France. http://meetings.espid.org/espid2010/abstracts/pdf/376.pdf (accessed Mar 2013).
- 20. Australian Bureau of Statistics. State and regional Indicators, Victoria, Jun 2010. Canberra: ABS, 2010. (ABS Cat. No. 1367.2.) http://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1367.2Jun+2010 (accessed Feb 2013).
- 21. Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine 2011; 29: 8279-8284.
- 22. Agorastos T, Chatzigeorgiou K, Brotherton JM, Garland SM. Safety of human papillomavirus (HPV) vaccines: a review of the international experience so far. Vaccine 2009; 27: 7270-7281.
- 23. Slade BA, Gee J, Broder KR, Vellozzi C. Comment on the contribution by Souayah et al., “Guillain-Barré syndrome after Gardasil vaccination: data from Vaccine Adverse Event Reporting System 2006-2009” [letter]. Vaccine 2011; 29: 865-866.
- 24. Poulos LM, Waters AM, Correll PK, et al. Trends in hospitalizations for anaphylaxis, angioedema, and urticaria in Australia, 1993–1994 to 2004–2005. J Allergy Clin Immunol 2007; 120: 878-884.
- 25. Mulla ZD, Lin RY, Simon MR. Perspectives on anaphylaxis epidemiology in the United States with new data and analyses. Curr Allergy Asthma Rep 2011; 11: 37-44.
- 26. Black S, Eskola J, Siegrist CA, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet 2009; 374: 2115-2122.
- 27. Brotherton JM, Deeks SL, Campbell-Lloyd S, et al. Interim estimates of human papillomavirus vaccination coverage in the school-based program in Australia. Commun Dis Intell Q Rep 2008; 32: 457-461.
- 28. Watson M, Shaw D, Molchanoff L, McInnes C. Challenges, lessons learned and results following the implementation of a human papilloma virus school vaccination program in South Australia. Aust N Z J Public Health 2009; 33: 365-370.
- 29. Farrington CP, Whitaker HJ, Hocine MN. Case series analysis for censored, perturbed, or curtailed post-event exposures. Biostatistics 2009; 10: 3-16.
- 30. Svanström H, Callréus T, Hviid A. Temporal data mining for adverse events following immunization in nationwide Danish healthcare databases. Drug Saf 2010; 33: 1015-1025.
- 31. Yih WK, Kulldorff M, Fireman BH, et al. Active surveillance for adverse events: the experience of the Vaccine Safety Datalink project. Pediatrics 2011; 127 Suppl 1: S54-S64.
- 32. Gold MS, Effler P, Kelly H, et al. Febrile convulsions after 2010 seasonal trivalent influenza vaccine: implications for vaccine safety surveillance in Australia. Med J Aust 2010; 193: 492-493. <MJA full text>





Abstract
Objectives: To determine background rates of potential adverse events following immunisation (AEFI) before expansion of the quadrivalent human papillomavirus (4vHPV) vaccination program to adolescent boys.
Design, patients and setting: Retrospective analysis of hospital discharge data obtained from the Victorian Admitted Episodes Dataset and emergency department visit data obtained from the Victorian Emergency Minimum Dataset for boys aged 12 to < 16 years during the period 1 July 2004 to 30 June 2009.
Main outcome measures: Numbers of and incidence rates for Guillain-Barré syndrome, anaphylaxis, seizures, syncope and other potential AEFI from 1 July 2004 to 30 June 2009, and estimated numbers of events after 4vHPV vaccination assuming no association (other than temporal) with the vaccine.
Results: We estimated background rates of neurological and allergic events in adolescent boys to be 252.9 and 175.2 per 100 000 person-years, respectively. Assuming an 80% vaccination rate with three doses per person — which equates to 1 440 000 doses administered nationally per year in the first 2 years of the program — about 2.4 episodes of Guillain-Barré syndrome would be expected to occur in the 6 weeks following vaccination. Within 1 day of vaccination, about 3.9 seizures, 0.3 episodes of anaphylaxis and 6.5 acute allergy presentations would be expected.
Conclusions: Routinely collected health outcome administration data can inform postlicensure safety surveillance of target conditions that might be perceived as AEFI.